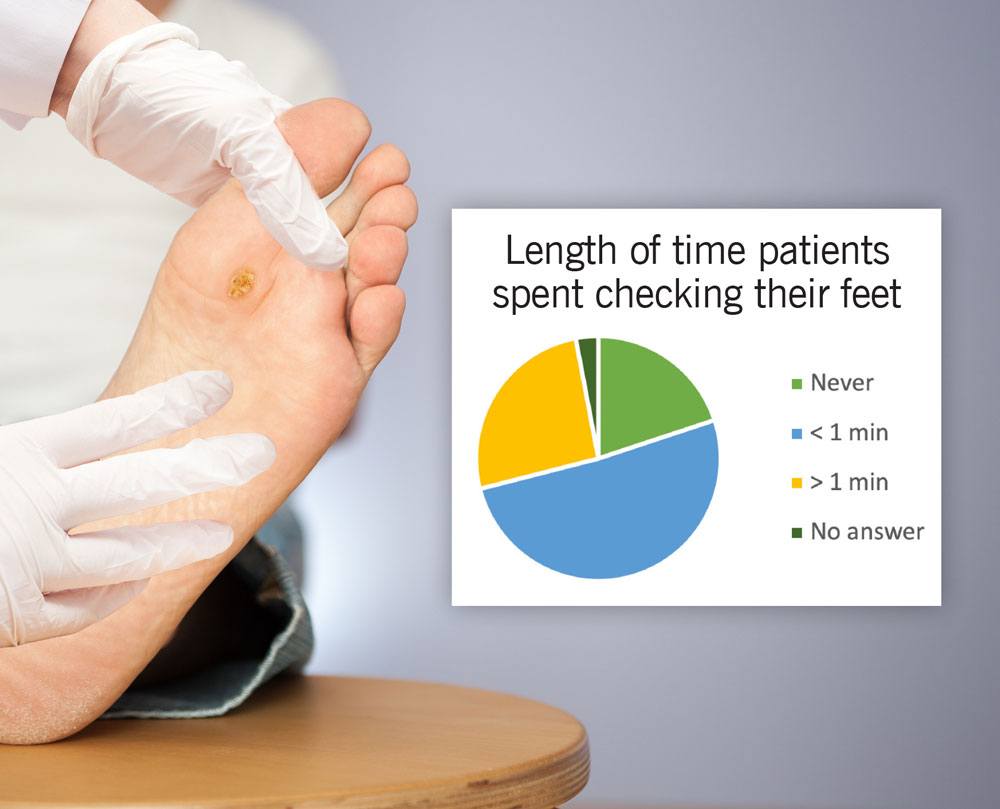
Figure 1. Rates of patient-based foot screening. Data from Wallace D, Perry J, Yu J, Mehta J, Hunter P, Cross KM. Assessing the Need for Mobile Health (mHealth) in Monitoring the Diabetic Lower Extremity. JMIR Mhealth Uhealth. 2019;7(4):e11879. Published 2019 Apr 16. doi:10.2196/11879
DFCON2019* showcased new technologies coming online to aid in the treatment and prevention of diabetic foot ulcers.
By Lynn Soban, PhD, MPH, RN
Diabetic foot ulcers (DFUs) are the most common complication of diabetes. Up to a quarter of patients with diabetes will suffer at least one DFU in their lifetime which can lead to amputation or death.
These wounds are notoriously challenging to prevent and treat. Factors that contribute to this medical challenge include:
- Prevention requires tight control of blood glucose.
- Decreased sensation in feet results in development of DFUs from innocuous causes such as an ill-fitting shoe or a crease in a sock.
- Poor patient adherence to evidence-based practices for prevention and treatment including performing daily foot exams and adhering to offloading (Figure 1).
- Poor guideline adherence by clinicians to consistently perform annual foot exams for diabetic patients, even for high-risk patients.
- Peripheral artery disease, a common comorbidity among people with diabetes, raises the risk of infection, non-healing ulcers, and amputation.
- Once a patient develops a DFU, recurrence is very common: 40% chance of recurrence within one year and 60% chance within 3 years.
- Only 75% of DFUs heal which makes the possibility of a lower extremity amputation (LEA) a real and devastating consequence of a DFU.
Between 2009 and 2015, CDC reported a staggering 50% increase in LEAs. Even more grim is the fact that 75% of those who undergo a LEA will not survive 5 years. These LEA rates speak to the need to double down on prevention and early identification efforts. Use of technology to improve DFU prevention and treatment is rapidly gaining momentum and has the potential to be a game-changer for this intractable problem.

Figure 2. The SmartMat is a remote temperature monitoring foot mat certified as a “high-traction” product by the National Floor Safety Institute and legally marketed in the USA as a class I medical Device. Photo is courtesy of the manufacturer, Podimetrics, Somerville, Massachusetts.
Wearable Sensors and Remote Monitoring
Current practices for prevention and treatment of DFUs are hindered by gaps in patient self-management. Wearable sensors, which are among the most popular technologies developed to manage chronic conditions, target barriers related to self-management. The development of wearable sensors to measure markers for development of DFU (temperature, oxygen saturation, and blood flow) has the potential to make the burden of self-management lighter and allow for early detection of DFUs.
“Adherence to offloading is a major reason for failure of DFU treatment”, said Bijan Najafi, PhD, Director at iCAMP (Interdisciplinary Consortium for Advanced Motion Performance) and Professor of Surgery, Baylor College of Medicine, Houston, Texas. “Sensors embedded in offloading devices such as shoe insoles are now able to provide real-time feedback and alerts to patients about their adherence to offloading; these can help us to tackle one of the most challenging barriers to DFU treatment.”
Another way that wearable sensors can revolutionize DFU prevention and treatment is through their use in remote monitoring. Increasingly, wearable sensors are able to transfer data to another machine through the use of cloud-based platforms such as Microsoft Azure. The availability of cloud-based platforms has made the computing resource requirements for remote monitoring available and affordable. This was no small feat: a major barrier to remote monitoring was the prohibitive cost of computing power.
“By allowing creators of applications to build, manage, and deploy their apps on a highly secure, scalable network, Microsoft Azure has the power to democratize startups for chronic disease management such as DFUs,” explained Bert Van Hoof of Microsoft.
It’s anticipated that wearable sensors and remote monitoring will be able to improve DFU prevention and treatment through several means:
- Improve adherence to daily foot checking by making it easier, faster, and more accurate which can lead to earlier detection of DFUs.
- Equip providers with remote monitoring capability to track patients more closely and detect DFUs at an earlier stage, possibly even before they are visible.
- Promote patient adherence to offloading and activity restrictions by providing real-time prompts to patients and caregivers, thus reducing healing time.
- Serve as educational devices that provide patients with insights into their disease management behaviors, thus reinforcing patient education.
- Increase patient engagement with prevention and treatment regimens through “gamification.”
- Facilitate communication between provider and patient (eg, about treatment plan, patient compliance, or need for office visit).
- Facilitate home care and decrease the need for hospitalization.
A variety of sensors specific to DFU detection are both under development and on the market.

Figure 3. MIMOSA, a MultIspectral MObile tiSsue Assessment device that uses near-infrared imaging to detect key indicators of tissue health (eg, oxygen saturation, blood circulation), provides a read-out that can be used to monitor and manage the development of diabetic foot ulcers. Photo is courtesy of the manufacturer, MIMOSA Diagnostics, Toronto, Ontario, Canada.
NEW TECHNOLOGY FOR DFU PREVENTION
SmartMat. Before diabetic foot ulcers develop, inflammation is present and can be detected by measuring foot temperatures. The daily remote temperature monitoring foot mat (Figure 2; SmartMat™; Podimetrics, Somerville, MA, USA) uses remote thermometry monitoring to measure plantar foot temperatures and transmits the data automatically to the Podimetrics care team, who triage any concerning findings and help patients receive appropriate, preventive treatments under the direction of their clinician. (See “Unilateral remote daily temperature monitoring to predict diabetic foot ulcers shows promise,” Lower Extremity Review, September 2019, pg 13-14.)
Features of SmartMat:
- Takes only 20 seconds to use and can help patients easily measure daily foot temperatures at home.
- Able to detect a DFU an average of 37 days before it presents which allows for early intervention.
- Equips providers with remote monitoring capability which allows them to more closely monitor patients, detect potential and actual DFUs earlier, and promptly initiate a care plan.
[Editor’s Note: The promise of this device for high-risk populations such as Veterans has resulted in early adoption of this device by the US Veteran’s Administration (VA). In December 2019 the VA announced that the Podimetrics SmartMat™ is in use at 15 VA medical centers and will be available to all veterans across the country through local Prevention of Amputations for Veterans Everywhere clinic providers.]
NEW TECHNOLOGY FOR DFU PREVENTION AND TREATMENT
MIMOSA. Another emerging technology, developed by Canadian physician researcher Karen Cross, PhD, MD, and her team at St. Michael’s Hospital in Toronto, is a state-of-the-art mobile health platform that uses optical imaging and artificial intelligence (AI) to non-invasively assess tissue health.
MIMOSA (which stands for MultIspectral MObile tiSsue Assessment device) uses near-infrared imaging to detect key indicators of tissue health such as the oxygen saturation of foot tissue and areas of poor circulation. This small and portable camera-device integrates with a smartphone and can image the skin with near infrared light. The resulting image (Figure 3) can be used in both the prevention and treatment of DFUs.
Cross’ impetus for creating MIMOSA is personal: a close relative lost a limb to diabetes. She underscored the potential impact this device can have, “Three million Canadians are living with diabetes—even more globally—and our device has the potential to help every single one of them.”
Features of MIMOSA:
- It is easy to use.
- The device does not touch the skin and will not interfere with wound healing.
- It can be used on a foot with active wounds to assess healing and can be used to monitor the non-affected foot during treatment to prevent development of a new wound.

Figure 4. Motus Smart Boot is an offloading device with interchangeable insoles (Optima Milliter boot) equipped with the Sensoria Core patient mobile app which provides continuous remote patient monitoring capabilities. Photo is courtesy of the manufacturers, Optima Molliter and Sensoria Health.
WEARABLE SENSORS TO HELP WITH OFFLOADING
Patients with an active DFU or those with a history of a DFU are often prescribed footwear or casts to reduce pressure on the affected area of the foot. Poor adherence to the offloading provided by these devices is a major reason for failure in wound management. During the first 2 weeks of healing walking just 1000 steps per day decreases the rate of healing by 5.2% a day.
Motus Smart Boot. Alberto Piaggesi, MD, from the University of Pisa, Italy, described the Motus Smart Boot (Figure 4), an offloading device equipped with sensors that monitor patient adherence to clinician recommendations on movement and provides continuous, remote monitoring of patients recovering from DFUs. This device is the result of a collaboration between Optima Molliter (Civitanova, Italy), a leading orthopedic medical footwear manufacturer, and Sensoria Health (Redmond, Washington) a leader in embedded sensors and mobile applications for remote monitoring.
The Motus Smart Boot is the first technology specific to DFU prevention and treatment that uses Microsoft Azure Cloud technologies. By applying AI algorithms to the sensor data, alert messages can be transmitted via text messages to patients, caregivers, and clinicians.
Features of the Smart Boot:
- Can be used for both prevention and treatment of plantar DFUs.
- Provides positive reinforcement and can alert patients to their non-compliance including time standing, walking, and wearing the device.
- Can be made non-removable, if desired.
- Efficacy is on par with total contact casting, the previous gold standard for DFU treatment.
- In addition to privacy protections, the Smart Boot integrates with HL7 interoperability standard-compliant hospital EMR systems.
Starting in 2020, Medicare will reimburse clinicians who use the Motus Smart Boot system to improve outcomes and reduce the risk of amputation. The coverage is approximately $122/member per month (See “Reimbursement for Remote Monitoring,” page 38).

Figure 5. Studies are in the works to see if e-tattoos may be used to promote wound healing in diabetic foot ulcers. Photo is courtesy of Nanshu Lu, PhD.
Microsoft’s Van Hoof presented a back-of-the envelope estimation of reimbursement for the Smartboot: “A primary care physician with an average number of Medicare patients could generate $200,000 per year if 20% of all eligible Medicare patients consented to remote monitoring.”
ON THE HORIZON
Chiara Daraio, PhD, of the California Institute of Technology is pioneering development of plant-based sensors that are stretchable and can be embedded in fabrics. Developed from pectin, a sugar molecule found in the cells of apples and other fruits, these devices capitalize on plants’ extraordinary sensitivity to temperature which results in a sensor that can measure and map temperature in real time. Given the importance of temperature monitoring in early detection and prevention of DFUs, plant-based sensors may someday be used in wearable devices to prevent DFUs.
Nanshu Lu, PhD, and a team of researchers at University of Texas are working on epidural electronics, also known as “e-tattoos”. These thin, light weight, graphene-based wearables are made of polyvinylidene fluoride, a polymer that’s capable of generating its own electric charge. E-tattoos are under pre-clinical trial for use in electrocardiograms (Figure 5). Another possible application of this technology is using e-tattoos to promote wound healing; by sending a microcurrent through the wound the e-tattoo could be used to kill bacteria, enhance protein synthesis and cell reproduction. This application has not yet been tested in humans, but lab work is underway and shows promise.
Reimbursement for Remote Monitoring
- Remote monitoring has the potential to extend patient care beyond hospital walls, to reduce hospital admissions, and to improve management of chronic diseases such as DFUs.
- Lack of reimbursement by insurance programs and health ministries has been a key barrier to availability and use of remote monitoring to prevent DFUs.
- As of January 1, 2020, the Centers for Medicare and Medicaid (CMS) will cover remote monitoring for devices including the MOTUS Smart Boot.
- Four CPT codes are available for remote patient monitoring (RPM):
✓ CPT code 99453: Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; set-up and patient education on use of equipment. (This covers onboarding a new patient onto an RPM service.)
✓ CPT Code 99454: Device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days. (This covers initial supply and daily recording or programmed alert transmission for a 30-day period for remote devices measuring the same physiologic factors as code 99453.)
✓ CPT Code 99457: Remote physiologic monitoring treatment management services, 20 minutes of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. (This covers remote monitoring and management of physiologic conditions, including 20 minutes per month of staff time requiring interactive communication with the patient or caregiver. This cannot be billed concurrently with 99091.)
✓ CPT Code 99458: Remote physiologic monitoring treatment management services, 20 minutes of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. (This can be used for an additional 20 minutes of staff time requiring interactive communication with the patient/caregiver.)
- Hopefully, these trends will spread to insurance companies and other public insurers so that use of remote monitoring will become more common.
*This article is based on presentations from the Diabetic Foot Global Conference—DFCon 2019, which was held in Los Angeles in October 2019. The session, Sensors & Common Sensibility: A Romantic Notion or a Game Changer, was moderated by Bijan Najafi, PhD, and David G. Armstrong, DPM, MD, PhD. Presentations by the following authors are highlighted: Karen Cross, MD, PhD; Alberto Piaggesi, MD; Nanshu Lu, PhD; Chiara Daraio, PhD; and Bert Van Hoof, Partner Group Manager of Azure IoT at Microsoft.