 Researchers and practitioners are gradually realizing that peak plantar pressure may not be as useful for predicting and preventing diabetic foot ulcers as previously thought, and are beginning to refocus their attention on plantar shear.
Researchers and practitioners are gradually realizing that peak plantar pressure may not be as useful for predicting and preventing diabetic foot ulcers as previously thought, and are beginning to refocus their attention on plantar shear.
By Metin Yavuz, PhD
Plantar shear and its relevance to diabetic ulceration have yet to be studied extensively, leading Dr David Armstrong to refer to these forces recently as the podiatric “dark matter” (see “Shear madness: beyond plantar pressure,” 2010 LER Resource Guide, page 12). This article will review the basic facts about shear forces under the foot and the preliminary studies conducted within the past years, in an attempt to bring light to Dr. Armstrong’s metaphor.
During human locomotion, ground reaction forces (GRF) act in all three dimensions under the foot. Many foot pathologies, in particular diabetic foot ulcers, have been associated with these mechanical factors. The two major components of GRF, namely vertical (normal) and horizontal (shear) forces, can easily be measured by a force plate. However, since force plates can provide only global force data not specific to any particular plantar region, their clinical value is limited. Plantar stresses, which require surface area information in order to be calculated, cannot be assessed with this type of equipment.
In materials science, the measure of stress, rather than force, is used to determine whether a material will fail when external forces are applied. Stress can be basically described as intensity of force, or average force per unit area. It is calculated by dividing the force value by the surface area on which the force acts.
To understand the difference between force and stress, think about an inflated latex balloon. If you apply a force of one pound on this balloon with your palm, chances are it will remain intact. However, if you apply exactly the same amount of force with a pin, you will most likely hear a loud “pop!” The difference between the two scenarios is the surface area on which the force is applied, hence the magnitude of force per unit area, or stress. Clearly, the surface area of the tip of a pin is much smaller than the surface area of the palm, and, consequently, the pin induces higher levels of stress. Therefore, utilizing stress magnitudes is more appropriate when material failure is of interest.
Like our balloon, the plantar skin is more vulnerable to the higher stresses that act on it. Because we cannot identify specific force concentrations with a force plate, devices have been developed to measure the vertical stresses (i.e., pressures) under the foot. Thanks to the availability of commercial pressure platforms and in-shoe systems, investigators have explored the association between foot pressures and ulcer occurrences in patients with diabetes. The initial plantar pressure studies revealed that higher foot pressures imposed higher risk for ulcers in patients with peripheral neuropathy.1-4 Later, however, especially in large-sample studies, it was seen that the sensitivity and specificity of foot pressure in determining ulceration were not as promising as first thought.5,6 Studies also revealed that patients with normal plantar pressure values may still ulcerate, whereas patients with elevated pressures may not.7,8 Moreover, in one of the very few prospective studies, ulcers were seen to occur at peak pressure sites in only 38% of patients.9 As a result, peak pressure has been labeled a “poor tool” for identifying patients at risk.6 Despite the report by Shaw and Boulton10 in 1996 that indicated a bigger role for impulse in the pathology of diabetic foot lesions, unfortunately, pressure-time integral has not been studied in a large cohort.
Various challenges in measurement of the other major component of the GRF have left researchers stuck with only one-dimensional (i.e., pressure) data for assessing foot abnormalities. As a result, plantar shear stresses have long remained a mystery in foot biomechanics, especially in diabetic foot research. Restriction to the readily available pressure data led to a tendency in the scientific community to oversimplify the etiology of diabetic foot ulcers and tie the pathology to pressure per se, even though an early study demonstrated the significance of shear.11
Quantifying shear

Figure 2. Peak pressure and shear locations can be at different sites under the foot. A representative diabetic case is shown here. Color and arrows represent pressure and shear, respectively. Data visualized by the FootVis software (Infoscitex, Waltham, MA).
The need for a validated and reliable system to quantify shear forces under the foot resulted in the development of The Cleveland Clinic’s pressure-shear platform (Figure 1). Between 2005 and 2007, we assessed a number of foot pathologies using this device.12-16 In our largest study, we have compared the distribution of plantar shear stresses in patients with diabetic neuropathy and healthy control subjects.13 Our research has revealed that the peak pressure and peak shear sites under the foot do not overlap in most patients (Figure 2). In fact, in 60% of the diabetic neuropathic patients, the site of maximum pressure and the site of maximum shear were separated by more than an inch.12 This finding has the potential to explain why ulcers do not necessarily develop at peak pressure locations. Surprisingly, peak pressure and shear locations did not overlap in only 35% of the control subjects. We have also observed plantar shear values in diabetic patients that were significantly higher than the corresponding values in healthy control subjects. The difference in certain shear variables was as great as 132%. On the other hand, peak pressure in patients with diabetes was higher by only 23%, when we compared the subject groups.
Double time
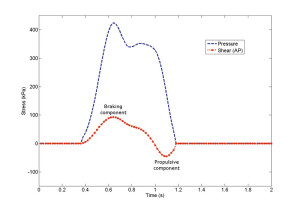
Figure 3. Pressure (blue) and anteroposterior shear (red) curves of a diabetic patient recorded by a single sensor of the device.
At the end of these studies, we also brought investigators’ attention to the application frequency of shear forces under the foot, which is twice that of the vertical force (i.e., pressure). This may be obvious when one considers the data set collected by a force plate during normal walking. The anteroposterior (i.e., fore-aft) and mediolateral shear curves obtained by a force plate usually present a peak and a valley, with positive and negative force values during a single step. This is because we experience both braking and propulsive forces under our feet in a single stance. However, since the data collected by a force plate come from the entire plantar surface, regional temporal characteristics of shear forces under the foot were still unknown. Our study has shown that the exact same region under the foot, such as the first metatarsal head, can experience anterior and posterior forces during a single step (Figure 3).13 We also observed that the mediolateral shear acted in a similar way.
The widely accepted foot ulceration theory states that “ulcers occur due to repetitive moderate stresses.”17 The fact that shear forces under the foot occur twice as frequently as vertical forces suggests that shear forces play a major role in ulceration despite lower magnitudes relative to pressure. The reason involves the principle of the “fatigue failure” mechanism.

Figure 4. A typical S-N curve for an arbitrary object. Coordinates on and above the curve indicate the material would fail at that stress-cycle combination. At the same stress magnitude, if the repetition count exceeds a threshold value, the material will fail.
Fatigue failure is defined as the progressive failure of an object due to repetitive loading. There are broadly two mechanisms that will cause a material to break down: macroscopic and microscopic. Tissue breakdown due to an acute trauma can be considered a macroscopic failure. In such a scenario, if the applied stress is higher than the strength of the material, the tissue will break down instantaneously. Whether the material will fail depends on the magnitude of applied stress. However, in the case of microscopic failure of a tissue, applied stresses can be much lower than the ultimate stress the material can endure. If these stresses act on the tissue repetitively for long periods of time, the microscopic damage will accumulate, and eventually the tissue will fail. The determinants of the breakdown in this case are the magnitude of stress and the count of cyclic loading (count of cycles is directly related to the application frequency and duration).
Stress-cycle curves
Researchers investigating fatigue failure of the soft tissues and other materials usually obtain data to draw stress-cycle (S-N) curves that assist in determining if and when the material will fail under different circumstances. A typical S-N curve can be found in Figure 4. It can be seen on this plot that different repetition numbers at the same stress value can either break the material down or leave it intact. Therefore, it is essential to realize the twofold difference in the repetition counts of shear and pressure under the foot when tissue failure is of concern. Unfortunately, to date there are no such data to draw S-N curves for the plantar tissue. Although such experiments may not be conducted on living tissue (i.e., patients with diabetes), cadaver and/or animal studies may shed some light on this subject.
Shear forces on or under the foot are also thought to cause hyperkeratosis and blisters. Since
calluses in diabetic patients usually precede ulcers, they can be perceived as early warning signs.18,19 Once a plantar callus develops under the foot, it acts as a stress riser. The effect is similar to having a pebble within the shoe and stepping on it, which will cause pain in a healthy individual due to elevated stresses at that region. This is primarily why we see peak pressures at callus sites; the callus formation leads to higher pressure. However, the relationship between calluses and elevated pressure does not necessarily mean that calluses occur because of high pressures. In fact, in studies conducted as early as the 1950s, it was shown that hyperkeratosis forms in the presence of frictional shear forces.20,21 Therefore, investigation of shear forces in this regard is also crucial to better understand the etiology of ulceration and minimize plantar ulcers.
During my career as a foot biomechanist, I have come across a critical question to which I was not able to find an exact answer: How do we reduce shear? As we know, human beings need shear forces to be able to walk and run, so what would happen if we could eliminate or minimize it? It is a known fact that diabetic pressure insoles can lower peak pressure magnitudes by distributing pressure over a greater surface area. It may well be possible to decrease peak shear stress by taking a similar approach. In these scenarios, use of orthotics does not alter the net vertical (other than the impact force during heel strike) or shear force under the whole foot. Interestingly, while pressure insoles cannot reduce the body weight of an individual, it may be feasible to reduce the net shear force under the foot via different mechanisms. But before addressing this concept, I would like to present an example of how shear reduction may function.
What do you experience when you drive on snow or ice? Readers from the southern states may not have any experience with such a challenge, but one likely answer is skidding. Even though the tires of the car keep revolving, they cannot traverse much distance because of diminished friction. I believe the same idea applies to human locomotion. If it were possible to reduce shear forces under an individual’s foot, this person would take shorter steps. In fact, researchers have studied the relationship between step length and propulsive shear forces under the foot and have shown a strong correlation between peak shear and step length.22-24 Therefore, it should be safe to say that we will take shorter steps if shear forces under the foot are decreased. In patients with diabetes, this relationship imposes a dilemma. On the one hand, by decreasing the net plantar shear force, incidence of foot ulcers may also be lowered. On the other hand, shear reduction would be possible at the expense of increasing the count of repetition, as one would need to take more steps to cover a certain distance. Since we still do not know what combinations of stress magnitudes and repetition counts cause skin breakdown, it is hard to determine if shear reduction would be beneficial at all.
Currently, there are a few orthotic devices available that claim to reduce the net shear force under the foot, employing different methods.25 We have recently evaluated the in vivo biomechanical efficacy of two such shear-reducing insoles at the Biomechanics Lab of the Ohio College of Podiatric Medicine. I will not go into the details of this study, as a manuscript is currently under review at another journal, but what we have found is that these insoles do not reduce shear as effectively in healthy volunteers as they do in vitro.26 There are a number of potential reasons for our findings, but I would like to emphasize that such orthotic designs need to go through a comprehensive set of in vitro and in vivo tests before they can be considered fully functional. Even if an orthotic device that effectively reduces shear forces within the shoe can be designed in the near future, the use of such insoles needs to be justified by addressing the magnitude-repetition paradoxical problem.
Complexity of response
I personally think that the skin, whether on the hand or under the foot, responds to mechanical forces in different ways, sometimes by forming a blister, other times a callus or ulcer. The type of the response depends on the ratio of shear to pressure, the repetition count, and the application duration, besides environmental and physiologic factors such as skin thickness, humidity, and temperature. The ratio of pressure to shear actually dictates whether one object will slide on another. Such sliding, even if on a small area and instantaneous, is thought to be a major deterministic factor in the structural integrity of the soft tissue, where it may impose a health risk. To predict how the skin responds to mechanical factors and when it will requires further research and elaboration. Until then, the podiatric dark matter will remain in the shadows.
Metin Yavuz, PhD, obtained his doctoral degree from the joint Applied Biomedical Engineering program of The Cleveland Clinic and Cleveland State University, under the supervision of Dr. Brian Davis. He is currently conducting research at the Ohio College of Podiatric Medicine, with a focus on the biomechanics of the diabetic foot.
Acknowledgment: The author would like to express his gratitude to Dr. Vincent Hetherington and Joani Lannoch of the Ohio College of Podiatric Medicine for providing support during the preparation of this article.
References
1. Boulton AJM, Hardisty CA, Betts RP, et al. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care 1983;6:26-33.
2. Frykberg RG, Lavery LA, Pham H, et al. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care 1998;21:1714-1719.
3. Ctercteko GC, Dhanendran M, Hutton WC, Le Quesne LP. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. Br J Surg 1981;68:608-614.
4. Stokes IAF, Faris IB, Hutton WC. The neuropathic ulcer and loads on the foot in diabetic patients. Acta Orthop Scand 1975;46:839-847.
5. Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? J Foot Ankle Surg 1998;37(4):303-307.
6. Lavery LA, Armstrong DG, Wunderlich RP, et al. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care 2003;26(4):1069-1073.
7. Hsi WL, Ulbrecht JS, Perry JE, et al. Plantar pressure threshold for ulceration risk using the EMEDSF platform [abstract]. Diabetes 1993;42(S1):103a.
8. Ledoux WR, Cowley MS, Ahroni JH, et al. No relationship between plantar pressure and diabetic foot ulcer incidence after adjustment for ulcer location. American Diabetes Association 65th Scientific Sessions (Abstract #67-OR), San Diego, June 2005.
9. Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 1992;35(7):660-663.
10. Shaw JE, Boulton AJM. Pressure time integrals may be more important than peak pressures in diabetic foot ulceration (Abstract). Diabet Med 1996;13(Suppl 7):S22.
11. Pollard JP, Le Quesne LP. Method of healing diabetic forefoot ulcers. Br Med J (Clin Res Ed) 1983;286(6363):436-437.
12. Yavuz M, Erdemir A, Botek G, et al. Peak plantar pressure and shear locations: relevance to diabetic patients. Diabetes Care 2007;30(10):2643-2645.
13. Yavuz M, Tajaddini A, Botek G, Davis BL. Temporal characteristics of plantar shear distribution: relevance to diabetic patients. J Biomechanics 2008;41(3):556-559.
14. Yavuz M, Davis BL. Regional analysis of plantar forefoot shear stress distribution in athletic individuals with a history of frictional blisters. J Am Podiatr Med Assoc 2010;100(2):116-120.
15. Yavuz M, Hetherington VJ, Botek G, et al. Forefoot plantar shear stress distribution in hallux valgus patients. Gait Posture 2009;30(2):257-259.
16. Yavuz M, Husni E, Botek G, Davis BL. Plantar shear stress profiles in rheumatoid arthritis patients: relevance to foot pain. J Am Podiatr Med Assoc. In press.
17. Hall OC, Brand PW. The etiology of the neuropathic plantar ulcer. J Am Podiatr Med Assoc 1979;69(3):173-177.
18. Delbridge L, Ctercteko G, Fowler C, et al. The aetiology of diabetic neuropathic ulceration of the foot. Br J Surgery 1985;72(1):1-6.
19. Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabet Med 1996;13(11):979-982.
20. Goldblum RW, Piper WN. Artificial lichenification produced by a scratching machine. J Invest Dermatol 1954;22(5):405-415.
21. MacKenzie IC. The effects of frictional stimulation on mouse ear epidermis. J Invest Dermatol 1974;63(2):194-198.
22. Martin PE, Marsh AP. Step length and frequency effects on ground reaction forces during walking. J Biomechanics 1992;25(10):1237-1239.
23. Orendurff MS, Bernatz GC, Schoen JA, Klute GK. Kinetic mechanisms to alter walking speed. Gait Posture 2008;27:603-610.
24. Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. J Biomechanics 2008;41(8):1639-1650.
25. Lavery LA, Lanctot DR, Constantinides G, et al. Wear and biomechanical characteristics of a novel shear-reducing insole with implications for high-risk persons with diabetes. Diabetes Technol Ther 2005;7(4):638-646.
26. Matassini M, Patel J, Ky T, et al. Do shear reducing diabetic insoles really reduce plantar shear? Diabetic Limb Salvage Conference 2009, Washington, DC, September 2009.









Excellent article with good information
Dr. Yavuz has provided one of the clearest explanations of how shear forces cause calluses, blisters, and ultimately diabetic foot ulcers. Dr. Yavuz raises this conundrum: friction causes shear and shear causes diabetic foot ulcers. However, friction is also necessary for efficient ambulation. The key is not to eliminate friction or shear from the entire plantar surface. Rather, one should offload shear only at problem locations. And, Dr. Yavuz has identified where those problem locations are: where calluses or previous ulcers have formed. By offloading friction and shear at areas of heavy callusing, ulcer formation can be prevented.
Thoroughly enjoyed this well written article. Understanding the interplay of shear forces with plantar pressure is not so straightforward. I do not think we should completely downplay the effects of increased plantar pressure. As an Institute that routinely uses the EMED plantar pressure system, still an association is seen with increased plantar pressure measurements, the presence of plantar callus and plantar neuropathic ulcerations. Having a plantar shear measuring system in the office would be welcomed, too!
In terms of addressing shear, do you concur that close contact casting is the optimum method of management (fibre-glass tcc)?