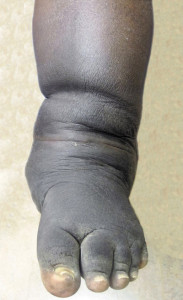
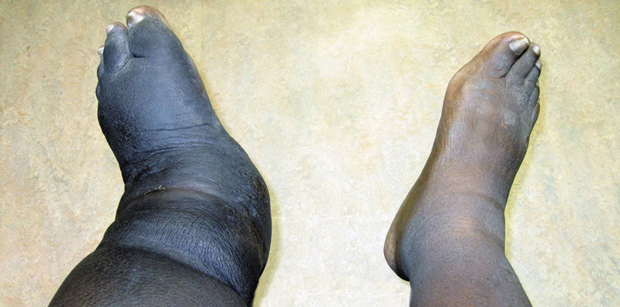
Figure 1. Clinical photograph of Charcot foot demonstrating foot and leg edema after removal of an Ace bandage.
Early detection of Charcot neuroarthropathy is critical for preventing the bone and joint destruction associated with later stages, but symptoms that mimic other conditions can make a differential diagnosis difficult.
By Georgeanne Botek, DPM, and Gina Hild, DPM
Charcot neuroarthropathy (CN) is a condition that is thought to be relatively rare, with epidemiological estimates occurring in 0.1% to 0.3% of patients with diabetes,1,2 although in the high-risk diabetic patient this value can exceed 13%.3 This diagnosis is one that is frequently misdiagnosed4 or undiagnosed in approximately 25% of cases5 prior to receiving a correct diagnosis at a referring institution. Detecting arthropathy at an early stage is imperative to prevent the downward spiral of fragmentation, fracture, and collapse of the structural support system of the lower extremity.4
CN was originally described in 1831 by John Mitchell Kearsley, who described lower limb denervation leading to bone and joint destruction in 47 cases within two separate articles.6,7 Jean-Martin Charcot, a French neurologist, then described the disorder as a complication of tabes dorsalis in 1868.9 His research was the first to provide a histopathological and clinical description of CN, and the international recognition he received resulted in the disorder being named after him.9 William Reilly Jordan was next to describe CN and its relationship with diabetic neuropathy in 1936,10 associating it with what is now known to be one of the most common causes of CN.
Less common causes of CN exist including spina bifida, cerebral palsy, meningomyelocoele, syringomyelia, leprosy, and alcohol abuse.9 Simultaneous kidney-pancreas transplant has been associated with CN;11 highly active anti-retroviral therapy (HAART), osteomyelitis, and surgery have also been implicated as causative factors.12,13 Vitamin B deficiency, amyloidosis, idiopathic sensorimotor neuropathy, infection, pernicious anemia, poliomyelitis, and prolonged corticosteroid use are other precipitating factors that are mentioned within the literature.3 This list is not exhaustive, and anything that is implicated with neuropathy can predispose an individual to CN.
Two traditional theories exist regarding the pathogenesis of Charcot’s disease. A neurovascular, or French, theory8,14 suggests that autonomic neuropathy leads to increased blood flow, resulting in a “washing out” of the bone with subsequent osteopenia, resorption, and fracture. Virchow and Volkmann challenged the French idea and proposed a neurotraumatic, or German, theory suggesting that individuals with neuropathic changes undergo a traumatic event, which progresses to an inflammatory response.14,15 The insensate foot fails to respond or feel pain as a “normal” individual’s foot would. As a result, continued pressure and repetitive trauma cause destructive changes within the bones and joints of the foot. Current thinking suggests that CN likely arises from a combination of both neurovascular and neurotraumatic etiologies.14,15
Newer theories have emerged that more accurately describe the pathogenesis of this condition. A triggering traumatic event in the face of neuropathy results in inflammation. Pro-inflammatory cytokines such as interleukin IL-1b and tumor necrosis factor alpha (TNF-a) are released. These factors are known to stimulate RANK-L (receptor activator of nuclear factor kappa-B ligand) which induces maturation of osteoclasts. Inflammatory cycles typically result in pain, which in a sensate individual would lead to splinting to protect the limb and induce healing. Neuropathic individuals instead continue to walk on their injured limbs, resulting in a continuum of inflammation and cascading of osteoclastic function, leading to eventual bone loss.16, 17
Clinical presentation
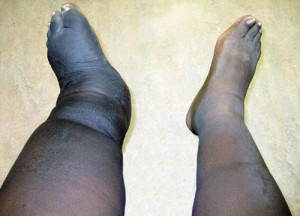
Figure 2. Clinical photograph of bilateral lower limbs demonstrating increased foot and leg edema on the left side, consistent with Charcot neuroarthropathy.
CN will be seen primarily in the diabetic population; however, keep in mind that any disorder that causes neuropathy can predispose an individual to the development of CN. Neuropathic arthropathy will present equally in men and women,3 more often in the fifth decade among those with type 1 diabetes and in the sixth decade among those with type 2 diabetes.18
Individuals with CN in the acute phase will typically present with a red, hot, swollen foot.4 Edema and erythema are usually present, and unilateral presentation is more common than bilateral presentation. Although CN can occur within any joint of the foot, the midfoot is the most common location.19 A study by Herbst 20 found that 50% of Charcot cases occurred in the midfoot, 28% in the hindfoot, 19% in the ankle and 3% in the forefoot. Other studies also suggest the midfoot presentation is most common, but ranges in distributions exist.21,22
Temperature changes will be noted in the affected limb, which has been verified by limited thermographic study.23 Recording the skin temperature differential between limbs on the initial presentation and all follow up visits may help the practitioner determine staging and progression of CN, but to our knowledge, a study has not been performed to confirm this. Temperature differences between limbs will return to zero when the acute phase of CN is complete. This diagnostic tool may allow the clinician to assess whether to transition the affected foot from acute therapy, such as total contact casting, into more conservative, chronic therapies, such as CROW walkers or protective diabetic footwear with custom bracing or custom molded inserts.
Neuropathy will also be seen in all patients with Charcot. Neuropathy can be assessed with the 5.07/10 g Semmes Weinstein monofilament (SWMF) examination, an evidence-based predictor of the likelihood that an individual will develop ulcerations or require a future amputation. Testing is simple and involves the application of the monofilament wire to various locations on the plantar and dorsal aspects of the foot. The monofilament should be applied until it buckles, which is considered 10 g of force. Patients may not sense the wire when it touches them, which would confirm a diagnosis of neuropathy. Maximum sensitivity and specificity for diabetic peripheral neuropathy is determined by a three-site test that includes the plantar hallux as well as the plantar third and fifth metatarsals.24
Chronic phases of CN will present with a temperature that is more similar to the unaffected contralateral limb. A foot in the chronic phase should no longer be edematous and erythematous. Radiographs will show evidence of bony consolidation and sclerosis will be resolving. Significant deformities may be present, depending on the effectiveness of treatment (if any) prior to evaluation. “Rocker bottom” feet typically present as the midfoot collapses, and ulcerations, especially on the plantar aspect of the midfoot, are common.
Differential Diagnosis of CN
A number of differential diagnoses may preclude the practitioner from immediately diagnosing CN. Infection, or osteomyelitis, is one of the main alternative diagnoses and is one of the most difficult to differentiate. Fracture, cellulitis, deep venous thrombosis (DVT), gout, ankle sprain, and septic joint are also differential diagnoses that can usually be ruled out with an adequate history and physical examination.
Infection such as cellulitis, septic arthritis, or osteomyelitis can usually be rejected as a differential diagnosis if an open ulceration is not present and traumatic inoculation did not occur.25 Laboratory studies including complete blood count (CBC), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) should be completed. If the results of these tests are unremarkable, infection and/or cellulitis is much less likely.21,26 However, the clinician must keep in mind that these tests may be less reliable in the diabetic population. Scintigraphic studies can be performed and will typically require the addition of indium labeling for accurate diagnosis.27 Magnetic resonance imaging can also be utilized to evaluate for osteomyelitis/cellulitis and will be discussed later in this article.
Deep venous thrombosis (DVT) will present with an enlarged limb and will be seen in a patient experiencing a hypercoagulable state as a result of genetics, surgery, age, immobilization, medications, or metastatic disease. This patient will experience pain in the posterior leg when pressure is applied to medial and lateral aspects of the calf. Tightness and fullness of the posterior aspect of the calf is usually noticed.
Well’s criteria can be utilized to determine those most at risk for DVT.28, 29 In this system, +1 point is given to those most at risk, such as those with cancer, paralysis or immobilization of more than three days, tenderness along course of deep veins, leg swelling, pitting edema, prior DVT, and collateral superficial veins. Two points are subtracted if another diagnosis is more likely than or as likely as DVT. Points are tallied and a high probability of DVT is associated with a score of more than 3, moderate probability if the score is 1 or 2, and low probability if a 0 score is obtained. Research suggests that duplex ultrasound will be positive in 71% of those individuals with high probability,28 and D-dimer can typically rule out this differential in low probability patients.28
Gout is the result of deposition of monosodium urate crystals within a joint; it typically occurs in the first metatarsophalangeal joint but can present in any joint of the foot. Those suffering from hypertension are three times more likely to develop gout. Thiazide diuretics, stress, surgery, infection, pneumonia, stroke and myocardial infarction have all been implicated as potential triggers. Elevated uric acid levels can be seen but are not diagnostic. Monoarticular flares are seen in 90% of individuals. Patients will describe pain that develops very acutely over a period of six to 12 hours. Untreated, these attacks will usually resolve in three to 14 days. Recurrent attacks can often take longer to resolve. Patients with normal sensation will relate that this condition is so painful they cannot even let the bed sheets touch their foot at night. This population will typically respond to non-steroidal anti-inflammatory drugs, colchicines, or corticosteroids, and a gradual improvement is noted within one to two weeks.30
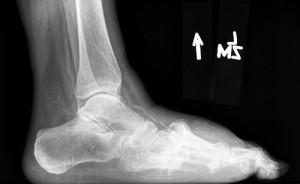
Figure 3. Weightbearing lateral radiograph demonstrating Charcot dislocation of the Lisfranc joint with dorsal subluxation and increased soft tissue edema.
Ankle sprains are one of the most common injuries presenting to the emergency department, second only to knee injuries. A patient with an ankle sprain will present with a history of trauma, which can potentially be the inciting event for the development of Charcot neuropathy. Sprains will present with tenderness to the medial or lateral ligamentous areas that house the ankle joint. Lateral ligamentous injuries are more common than medial. Diffuse pain may be noted if significant swelling is present. Radiographic evaluation can rule out underlying ankle fracture. MRI can evaluate the ligaments of the ankle and rule out this differential diagnosis if needed.31
Septic arthritis is potentially one of the most dangerous differential diagnoses on this list to miss. This is a true medical emergency. Infected joints are usually monoarticular and severely painful, and will present similar to gout. However, individuals with septic arthritis will present clinically with fever and additional constitutional symptoms (by comparison, systemic responses are only occasionally seen in cases of gout). Inoculation, via direct or more commonly hematogenous extension, is necessary for infection to occur. In 50% of cases, a bacteremia will be noted.
Diagnosis should be made quickly through an accurate and thorough clinical exam, and definitive treatment should not be delayed while waiting for laboratory results to be completed. Intravenous antibiotics are initiated with both gram-positive and gram-negative coverage. Joint aspiration with synovial fluid analysis for gram stain and culture are performed in order to tailor antibiotic coverage. ESR and CRP levels may be elevated. A septic joint left untreated can deteriorate within days, and long term or permanent disability will ensue.32
Radiographic Imaging
The Eichenholtz classification system is the one most frequently utilized when evaluating neuroarthropathy. Eichenholtz developed a series of three radiographic stages to describe the progression of change within the neuroarthropathic foot .33 Stage 1, termed the “stage of development”, is characterized radiographically by fragmentation of articular cartilage and subchondral bone. Debris is noted within articular margins and joint dislocation may be seen. Stage 2, termed the “stage of coalescence”, is characterized radiographically by absorption of debris, fusion of larger fragments, and sclerosis of bone ends. Stage 3, termed the “stage of reconstruction”, is identified by the reformation of joint architecture. Fragments are now rounded and sclerosis is minimized as revascularization occurs.14, 25, 33
Yu14 was one of the first to describe the entity known as the “stage 0” Charcot foot. An individual in stage 0 will present with an acute, red, hot, swollen foot and bounding pulses. Pain may or may not be noticed. Radiographic presentation may include a simple or comminuted fracture but may also be normal in some. Subtle widening of the joint spaces or dislocation may be discovered. Yu stated that once fragmentation or osteopenia was noted on clinical radiographs, the condition had progressed from stage 0 to stage 1. Yu also emphasized the need to identify the individual with stage 0 in order to prevent the sequelae of undiagnosed Charcot.
Bone scintigraphy
Early changes, such as those associated with stage 0 Charcot, can reliably be observed with triphasic bone scan. Some studies even indicate that abnormalities could be seen with a technetium-99 bone scan months before clinical or radiographic abnormality becomes apparent.34 Uptake will be noted in all three phases. If osteomyelitis is suspected, osteomyelitis indium 111 labeled or HmPAO scan can be obtained. Osteomyelitis should not be confirmed until a positive 24 hr follow up reading is obtained; negative results at three to six hours can rule out osteomyelitis.14
Magnetic Resonance Imaging
Valuable information about the Charcot foot can be obtained with MRI. The benefits of MRI over plain radiographs include a more rapid determination of bone stress injury, which typically precedes fracture in these individuals and can lead to diagnosis at the stage 0 phase. “Bone edema” is seen very clearly on MRI and is typically what a clinician looks for when evaluating for neuroarthropathy, whereas radiographs and bone scans cannot reveal this information.35 Fracture lines will follow bone edema if the pathology is not discovered and the neuropathic patient continues to bear weight.35 Additional early pathological findings seen on MRI include marrow enhancement in subchondral areas and peri-articular edema. With more significant destruction, cysts, erosions, and bone fragmentation will be seen. Because CN is primarily an articular disorder, subchondral distribution will typically be seen as well. Multiple joints are typically affected and the most commonly affected are the tarsometatarsal and midfoot articulations.27,36,37
It can still be difficult to differentiate infection from arthropathy based on MRI evaluation alone, as bone edema and osteomyelitis can have a similar appearance.38 It is, therefore, important to carefully evaluate MRI studies for subtle differences that can lead to a correct diagnosis. Infection is favored as the diagnosis if sinus tracts, subchondral cysts, bone marrow abnormalities that are diffuse in nature, or erosions of the bone are noted. Diagnosis is determined by low signal intensity on T1 weighted images within the bone itself. A high, or hyperintense, signal is noted within the bone marrow on T2 weighted images.
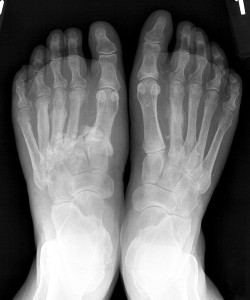
Figure 4. Lateral subluxation of metatarsal bases 2-5 with fracture at 2nd metatarsal base and slight medial dislocation of medial Lisfranc joint; obliteration of Lisfranc joint space.
It is important to remember that infection will be caused by contiguous spread from ulceration or surrounding cellulitis; osteomyelitis in the absence of these entities is extremely rare. Osteomyelitis will more commonly be seen in areas that are typically prone to ulceration such as the digits, metatarsal heads, calcaneus, and malleolus. In the presence of infection, a more localized involvement and cortical breaks are visualized.27,36,37 Localized abscess formation can also be seen as a contiguous source in osteomyelitis. If abscess is suspected, gadolinium should be added to the MRI study. This will allow for rim enhancement of abscess and better visualization.31
Cellulitis will be seen as a low signal in the soft tissues surrounding the bone on T1-weighted images and hyperintensity seen on T2-weighted images. Gadolinium will also enhance areas of soft tissue involvement when reviewed on MRI.
FDG Positron Emission Tomography
FDG-PET (2-fluoro-2deoxy-glucose positron emission tomography) scanning offers some very distinct advantages over MRI in relation to Charcot neuroarthropathy. PET scans can differentiate infection from CN on the basis of glucose metabolism; infection will result in a higher uptake of glucose, which can be measured. Charcot disturbances typically have standardized uptake values (SUV) of approximately 1.8, whereas osteomyelitis will result in much higher SUVs, usually in the range of 3 to 7.38 FDG PET scans produce a focal, distinct area of uptake in an individual with osteomyelitis and a more diffuse uptake in an individual with Charcot neuropathy allowing the practitioner to distinguish between the two diagnoses.
PET scans have another distinct advantage in that patients with metal implants can be reliably evaluated with this technique without complicating artifacts to obscure views, which can occur with MRI. Ring PET has been found to be more reliable than hybrid (dual head gamma camera) PET in at least one study,39 since the resolution of ring PET scans is higher than hybrid PET scans. Ring FDG PET offers a higher sensitivity and specificity in differentiating Charcot foot from infection when compared to MRI results. 38, 39
FDG PET is rarely utilized clinically in the diagnostic workup of Charcot neuroarthropathy today. Limitations of this imaging modality include its significant expense, which can range from $2,000 to $8,000 depending on the institution. Ionizing radiation exposure and limited availability of this technology outside of a tertiary care setting are also limitations that can preclude its routine use. Time will tell if this diagnostic modality’s usage will increase but certainly it may be, pending future improvements in cost effectiveness, accessibility, and increasing awareness and education of practitioners.
Conclusion
Although many confounding differential diagnoses exist, Charcot arthropathy can be relatively simple to diagnose if the practitioner has an understanding of the condition and a high index of suspicion. When a diabetic, insensate individual presents without an open ulceration and has a red, hot, swollen foot (especially the midfoot) with bounding pulses, Charcot arthropathy should be at the top of the practitioner’s list of differential diagnoses. Multiple diagnostic tools are now available to help differentiate CN from osteomyelitis, including MRI and FDG- PET. FDG-PET may be particularly useful in the individual with metal implants.
Although treatment of CN is beyond the scope of this article, it is worth noting that if a practitioner comes across an individual with suspected CN, implementing non-weight bearing status is of utmost importance. The patient should be placed in a posterior splint or total contact cast and given crutches or a wheelchair to ensure that no additional pressure is applied to the foot. Protecting the limb will prevent further breakdown of the neuroarthropathic joint and allow the healing process to begin. A referral to a foot and ankle specialist equipped to deal with this population should be made as quickly as possible.
Disclosure
Neither of the authors have any potential conflicts of interest to disclose.
References
1. Sinha S, Munichoodappa CS, Kozak GP. Neuro-arthropathy (Charcot joints) in diabetes mellitus (clinical study of 101 cases). Medicine 1972;51(3):191-210.
2. Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care 2000;23(6):796-800.
3. Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008;25(1):17-28.
4. Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994;15(5):233-241.
5. Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int 1994;15(5):233-241.
6. Mitchell J. On a new practice in acute and chronic rheumatism. Am J Med Sci 1831;8:55-64.
7. Mitchell J. Further cases and observations relative to rheumatism. Am J Med Sci. 1833;12:360.
8. Charcot J. Sur quelques arthropathies qui paraissent dependre d’une lesion du cerveau ou de la moelle epiniere. Arch Physiol Norm Pathol 1868;1:161-178.
9. Molines L, Darmon P, Raccah D. Charcot’s foot: newest findings on its pathophysiology, diagnosis and treatment. Diabetes Metab 2010;36(4):251-255.
10. Jordan W. Neuritic manifestations in diabetes mellitus. Arch Intern Med 1936;57:307-366.
11. Matricali GA, Bammens B, Kuypers D, et al. High rate of Charcot foot attacks early after simultaneous pancreas-kidney transplantation. Transplantation 2007;83(2):245-246.
12. Ndip A, Jude EB, Whitehouse R, et al. Charcot neuroarthropathy triggered by osteomyelitis and/or surgery. Diabet Med 2008;25(12):1469-1472.
13. Oh-Park M, Dove C, Sheskier S, Sheehan P. Charcot neuroarthropathy in the era of HAART. Lancet 2006;367(9506):274.
14. Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot’s neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc 2002;92(4):210-220.
15. Armstrong DG, Peters EJ. Charcot’s arthropathy of the foot. J Am Podiatr Med Assoc 2002;92(7):390-394.
16. Jeffcoate W. The causes of the Charcot syndrome. Clin Podiatr Med Surg 2008;25(1):29-42.
17. Jeffcoate W, Lima J, Nobrega L. The Charcot foot. Diabet Med 2000;17(4):253-258.
18. Petrova NL, Foster AV, Edmonds ME. Difference in presentation of charcot osteoarthropathy in type 1 compared with type 2 diabetes. Diabetes Care 2004;27(5):1235-1236.
19. Sanders LJ, Frykberg RG. The Charcot foot. In: Frykberg RG, ed. The high risk foot in diabetes mellitus. New York: Churchill Livingstone; 1991:325-335.
20. Herbst SA, Jones KB, Saltzman CL. Pattern of diabetic neuropathic arthropathy associated with the peripheral bone mineral density. J Bone Joint Surg Br 2004;86(3):378-383.
21. Pakarinen TK, Laine HJ, Honkonen SE, et al. Charcot arthropathy of the diabetic foot. Current concepts and review of 36 cases. Scand J Surg 2002;91(2):195-201.
22. Schon LC, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop Relat Res 1998(349):116-131.
23. Sandrow RE, Torg JS, Lapayowker MS, Resnick EJ. The use of thermography in the early diagnosis of neuropathic arthropathy in the feet of diabetics. Clin Orthop Relat Res 1972;88:31-33.
24. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination is a significant predictor of the risk of foot ulceration and amputation in patients with diabetes mellitus. J Vasc Surg 2011;53(1):220-226.
25. Botek G, Anderson MA, Taylor R. Charcot neuroarthropathy: An often overlooked complication of diabetes. Cleve Clin J Med 2010;77(9):593-599.
26. Judge MS. Infection and neuroarthropathy: the utility of C-reactive protein as a screening tool in the Charcot foot. J Am Podiatr Med Assoc 2008;98(1):1-6.
27. Ledermann HP, Morrison WB. Differential diagnosis of pedal osteomyelitis and diabetic neuroarthropathy: MR imaging. Semin Musculoskelet Radiol 2005;9(3):272-283.
28. Hotoleanu C, Fodor D, Suciu O. Correlations between clinical probability and Doppler ultrasound results in the assessment of deep venous thrombosis. Med Ultrason 2010;12(1):17-21.
29. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350(9094):1795-1798.
30. Quillen DM. Crystal arthropathies: recognizing and treating “the gouch.” Prim Care 2010;37(4):703-711.
31. Abbassian A, Thomas R. Ankle ligament injuries. Br J Hosp Med 2008;69(6):339-343.
32. Berendt T, Byren I. Bone and joint infection. Clin Med 2004;4(6):510-518.
33. Eichenholtz S. Charcot joints. Springfield, IL: CC Thomas; 1966.
34. Classen JN, Rolley RT, Carneiro R, Martire JR. Management of foot conditions of the diabetic patient. Am Surg 1976;42(2):81-88.
35. Chantelau E, Richter A, Schmidt-Grigoriadis P, Scherbaum WA. The diabetic charcot foot: MRI discloses bone stress injury as trigger mechanism of neuroarthropathy. Exp Clin Endocrinol Diabetes 2006;114(3):118-123.
36. Tan PL, Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol 2007;80(959):939-948.
37. Ahmadi ME, Morrison WB, Carrino JA, et al. Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR imaging characteristics. Radiology 2006;238(2):622-631.
38. Basu S, Chryssikos T, Houseni M, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun 2007;28(6):465-472.
39. Hopfner S, Krolak C, Kessler S, et al. Preoperative imaging of Charcot neuroarthropathy in diabetic patients: comparison of ring PET, hybrid PET, and magnetic resonance imaging. Foot Ankle Int 2004;25(12):890-895.







Nice informative blog about Char cot neuroarthropathy. It gives some idea about Charcot neuroarthropathy. Thanks for sharing this information.