 New research suggests lower extremity clinicians should consider implementing gait training in combination with targeted strengthening of the peroneus longus and gluteus medius muscles to help restore normal gait patterns in patients with chronic ankle instability.
New research suggests lower extremity clinicians should consider implementing gait training in combination with targeted strengthening of the peroneus longus and gluteus medius muscles to help restore normal gait patterns in patients with chronic ankle instability.
By Rachel Koldenhoven, MEd, ATC
Lateral ankle sprains are among the most common musculoskeletal injuries, not only in the physically active population1 but also in the general population. Potential risk factors for sustaining a subsequent ankle sprain include a history of lateral ankle sprain,2 poor postural control,3 and an inverted foot position prior to heel contact during gait.4,5 Regardless of the high prevalence of the injury, many individuals (40%-55%) do not seek medical attention following an initial lateral ankle sprain.6,7 This lack of treatment may result in many long-term consequences, such as decreased quality of life,8 decreased physical activity across the individual’s lifetime,9,10 and even an early onset of ankle osteoarthritis.11,12
Following a lateral ankle sprain, 40% of individuals develop a condition known as chronic ankle instability (CAI); the 60% who do not develop CAI are known as copers.6 CAI is commonly characterized as having decreased self-reported function, repetitive bouts of instability or feelings of “giving way,” and residual symptoms for at least one year following an initial lateral ankle sprain.13 In general, copers are individuals who have a history of at least one lateral ankle sprain but do not have pain, weakness, or residual instability, and have returned to their normal activity level.
Many clinicians and researchers are aware that individuals who sustain lower extremity injuries tend to walk differently than healthy individuals who have never been injured. The foundation for our research stems from the basic understanding that individuals with CAI walk differently than individuals who have never sprained their ankle. Alterations and strategies adapted by CAI patients have been previously reported in terms of neuromuscular control,14,15 plantar pressure,15-17 kinematics,5,18-21 and spatial-temporal measures during gait.22 Clinicians should be aware that these alterations during gait exist and that they could potentially contribute to subsequent lateral ankle sprains or residual symptoms. It is important to first understand the general alterations that occur in individuals with CAI before attempting to intervene.
General discussion of study and findings
The purpose of our study23 was to simultaneously analyze muscle activation throughout the entire gait cycle and plantar pressure throughout the stance phase of walking gait in young adults with and without CAI. This was done to gain a more comprehensive understanding of the role of muscle activity and plantar pressure distribution in CAI gait pathomechanics. Previous studies have also looked at muscle activity, but the focus has been on analyzing muscle activity during the stance phase only, or a small time period around initial contact, but not throughout the entire gait cycle.4,15
We analyzed 17 individuals with CAI and 17 healthy individuals as they walked on a treadmill. Our primary outcome of interest was the medial-lateral location of the center of pressure (COP) at 10% intervals of stance. The location of the COP from 1% to 10% of stance was condensed into one data point that represented the average location of the COP at 5% of stance. We continued this process for the entire stance phase to create 10 discrete data points.

Figure 1. Medial-lateral location of COP throughout stance for healthy and CAI participants. MD = mean difference.
Our results revealed the COP of the CAI group was significantly more lateral than the control group throughout the entire stance phase. At initial contact, the location of the COP in the CAI group was about 3 mm more lateral on average. During midstance, the CAI group’s location of COP was more than 7 mm more lateral on average (Figure 1).
In addition to the lateral deviation throughout stance, we found the CAI group had higher average peak pressure and pressure-time integral in the lateral forefoot compared with the control group. This indicates the location of COP is not only more lateral throughout all of stance, but the forces are higher in the lateral forefoot in individuals with CAI. This could be problematic, because if individuals with CAI are walking more on the lateral aspect of their foot they will be closer to the edge of their lateral base of support. When the location of the COP falls outside that lateral base of support, a subsequent lateral ankle sprain or feelings of giving way may result. These findings may also help to explain why individuals with CAI demonstrate more rigid kinematic profiles during the stance phases of gait.18 Having a laterally deviated COP may limit the degrees of freedom available throughout stance and limit the ability of individuals with CAI to respond to subtle changes.
We also identified differences in muscle activity between the CAI and healthy groups. For this study, we analyzed the muscle activation for the tibialis anterior, peroneus longus, medial gastrocnemius, and gluteus medius muscles. We looked at the muscle activation amplitude throughout the entire stride cycle. The stride cycle was split into the stance (1%-60% of stride cycle) and swing (61%-100% of stride cycle) phases of gait. This was then broken down further into 10% intervals during stance (to match the location of the COP for plantar pressure) and 25% intervals during the swing phase of gait.
We found significantly higher gluteus medius muscle activation in the individuals with CAI than the controls from 50% of stance through the first 25% of the swing phase (Figure 2). This increase in gluteus medius activation could potentially be a proximal strategy used by CAI individuals to compensate for a distally supinated foot position. CAI patients have been shown to walk with a 43% wider base of support than healthy individuals, which is thought to be a method of improving dynamic stability that may reduce the demand on the static and dynamic stabilizers of the ankle.22 Our study is the first to identify altered gluteus medius muscle function during the gait cycle in individuals with CAI.
We also looked at the extent of activation for the time around initial contact by measuring the root mean square (RMS) amplitude for the 100 ms prior to initial contact (pre-IC) and the 200 ms following initial contact (post-IC). We identified significant differences between the two groups for only the 100 ms pre-IC (Figure 3). The CAI group demonstrated less anterior tibialis activation than controls, but greater activation for the peroneus longus, medial gastrocnemius, and gluteus medius muscles just prior to initial contact. These results may help explain the decrease in vertical foot-floor clearance and more plantar flexed position prior to IC demonstrated by individuals with CAI.5,24,25
Gait and CAI in the literature
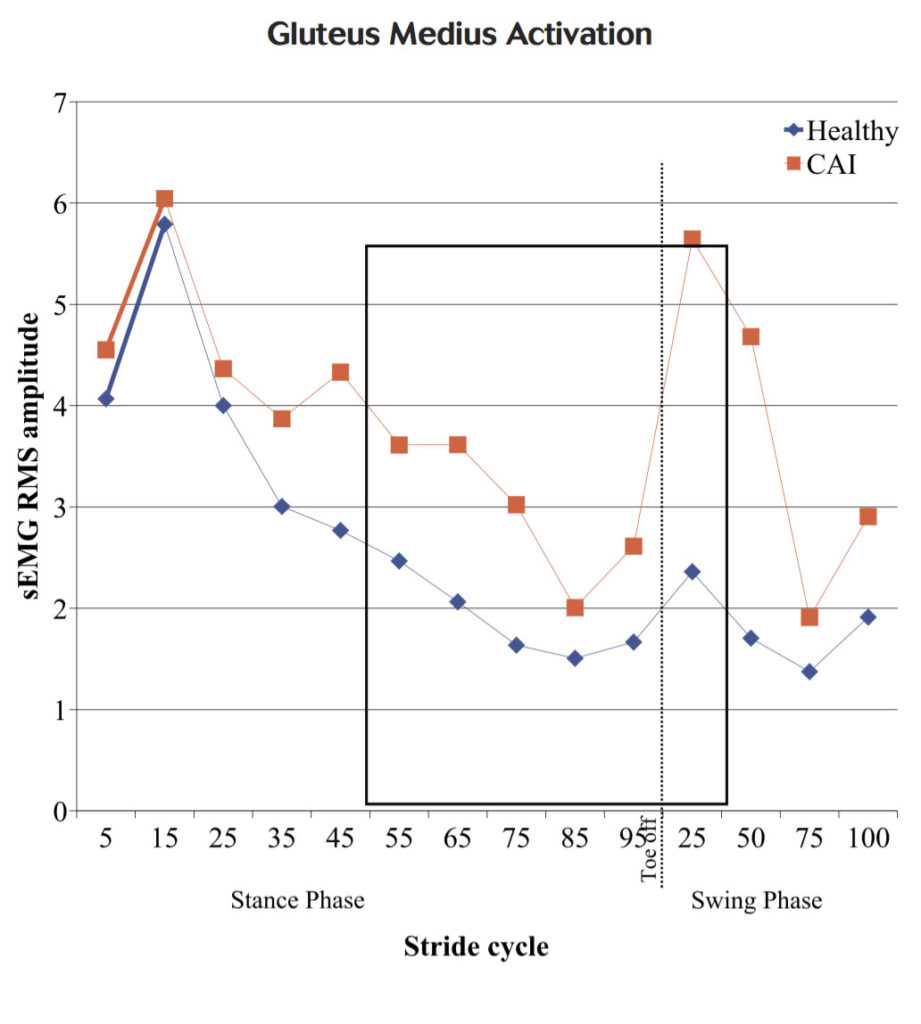
Figure 2. Gluteus medius surface electromyography root mean square (sEMG RMS) amplitude throughout entire gait cycle for healthy and CAI participants. Boxed area indicates significant differences between groups.
The peroneus longus (PL) muscle is commonly studied in the CAI population. The PL is not only used to actively evert the foot, but also plays a role in pronation during the stance phase of gait. The PL also provides a great amount of dynamic stability for the lateral ankle.26 In addition to our study, previous research has also identified alterations in the function of the PL in individuals with CAI. Feger et al14 suggested that “preactivation may be a coping strategy that is effective for normal ambulation and allows individuals with CAI to complete functional tasks, but it may be ineffective in providing adequate dynamic stability to the lateral ankle to prevent injury.” Our results are consistent with the hypothesis that the preactivation may not be effective, in particular our finding that plantar pressures were lateralized throughout stance.
Previous studies involving this population have also identified differences in kinetics and kinematics. During running, individuals with CAI have a lateral shift in plantar pressure of the rearfoot during initial contact and a greater lateral COP trajectory during loading compared with controls.27 During jogging, individuals with CAI have demonstrated increases in pressure located in the lateral midfoot and lateral forefoot.17 An inverted foot position pre-IC5 may manifest into the increased lateral loading27 and laterally deviated COP trajectory15 identified by previous studies and the lateral location of the COP throughout stance identified by our study.
Drewes et al28 also identified more inverted foot positioning in individuals with CAI than in controls, but was the first study to observe that individuals with CAI may have a less coordinated movement pattern with regard to alterations in kinematics and joint coupling during terminal swing. Additionally, two studies have reported that individuals with CAI have less variability in shank and rearfoot coupling than controls29 and less stride-to-stride variability during the stance phase.18 These findings could indicate that individuals with CAI have a more constrained sensorimotor system than healthy individuals and are less able to adapt to changes.
Clinical implications
While descriptive studies are important for understanding the general alterations between the groups presented, it is also important for clinicians and researchers to understand how a variety of interventions can be used to target those deficits. Several studies have used a variety of intervention strategies to target deficits seen in individuals with CAI.30–33 Donovan et al30 recently used auditory feedback as an intervention to shift plantar pressure medially, which caused an increase in PL and medial gastrocnemius activation following initial contact. The same group has also identified an increase in PL activation while walking associated with two different destabilization devices in individuals with CAI compared to healthy individuals.31
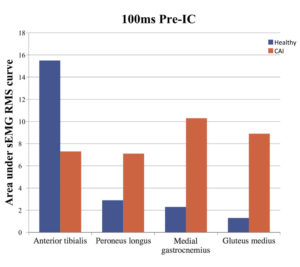
Figure 3. The area under the sEMG RMS curve 100 ms prior to initial contact for healthy and CAI participants.
Although Donovan et al used novel and innovative techniques, studies have also been conducted looking at more traditional intervention techniques commonly employed by clinicians. Chinn et al32 studied the immediate effects of using ankle tape for support in individuals with CAI and found decreased plantar flexion and inversion during the gait cycle compared with no tape, which suggests a more neutral foot position during walking. A similar study by Herb et al34 found that taping decreased the variability of coupled motion in both healthy and CAI groups. Barlow et al33 identified a decreased amplitude for the PL and delayed activation of the tibialis anterior, PL, rectus femoris, and gluteus medius muscles when individuals with CAI were wearing a brace during walking. Collectively, these findings suggest taping and bracing interventions may be beneficial for protecting individuals with CAI from future sprains.
Lastly, a study by McKeon et al35 studied the effects of a four-week progressive balance training program in individuals with CAI. The clinical take-home message was that the intervention “improves the control of the shank and rearfoot during walking, but not jogging in those with chronic ankle instability.”
Conclusions
Individuals with CAI demonstrate alterations during walking. Clinicians and researchers should understand these deficits and attempt to address them during rehabilitation. There are currently no evidence-based recommendations for gait retraining in individuals with CAI, but clinicians should be aware of distal and proximal neuromuscular strategies when attempting to improve gait pathomechanics in CAI patients. Clinicians should consider implementing gait training in combination with targeted strengthening of the peroneus longus and gluteus medius muscles to restore normal gait patterns.
Rachel Koldenhoven, MEd, ATC, is a doctoral student at University of Virginia, Department of Kinesiology – Sports Medicine, in Charlottesville.
Disclosure: No conflicts of interest or disclosures to report in relation to this review.
- Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. J Athl Train 2007;42(2):311-319.
- Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Athl Train 2002;37(4):376-380.
- McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord 2008;9(1):76.
- Delahunt E, Monaghan K, Caulfield B. Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am J Sports Med 2006;34(12):1970-1976.
- Monaghan K, Delahunt E, Caulfield B. Ankle function during gait in patients with chronic ankle instability compared to controls. Clin Biomech 2006;21(2):168-174.
- Doherty C, Bleakley C, Hertel J, et al. Recovery From a first-time lateral ankle sprain and the predictors of chronic ankle instability a prospective cohort analysis. Am J Sports Med 2016;44(4):995-1003.
- McKay GD, Goldie PA, Payne WR, Oakes BW. Ankle injuries in basketball: injury rate and risk factors. Br J Sports Med 2001;35(2):103-108.
- Hubbard-Turner T, Turner MJ. Physical activity levels in college students with chronic ankle instability. J Athl Train 2015;50(7):742-747.
- Arnold BL, Wright CJ, Ross SE. Functional ankle instability and health-related quality of life. J Athl Train 2011;46(6):634-641.
- Houston MN, Van Lunen BL, Hoch MC. Health-related quality of life in individuals with chronic ankle instability. J Athl Train 2014;49(6):758-763.
- Valderrabano V, Pagenstert G, Horisberger M, et al. Sports and recreation activity of ankle arthritis patients before and after total ankle replacement. Am J Sports Med 2006;34(6):993-999.
- Golditz T, Steib S, Pfeifer K, et al. Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthritis Cartilage 2014;22(10):1377-1385.
- Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Orthop Sports Phys Ther 2013;43(8):585-591.
- Feger MA, Donovan L, Hart JM, Hertel J. Lower extremity muscle activation in patients with or without chronic ankle instability. J Athl Train 2015;50(4):350-357
- Hopkins J, Coglianese M, Glasgow P, et l. Alterations in evertor/invertor muscle activation and center of pressure trajectory in participants with functional ankle instability. J Electromyogr Kinesiol 2012;22(2):280-285.
- Nyska M, Shabat S, Simkin A, et al. Dynamic force distribution during level walking under the feet of patients with chronic ankle instability. Br J Sports Med 2003;37(6):495-497.
- Schmidt H, Sauer LD, Lee SY, et al. Increased in-shoe lateral plantar pressures with chronic ankle instability. Foot Ankle Int 2011;32(11):1075-1080.
- Herb CC, Chinn L, Dicharry J, et al. Shank-rearfoot joint coupling with chronic ankle instability. J Appl Biomech 2014;30(3):366-372.
- Terada M, Bowker S, Thomas AC, et al. Alterations in stride-to-stride variability during walking in individuals with chronic ankle instability. Hum Mov Sci 2015;40:154-162.
- Chinn L, Dicharry J, Hertel J. Ankle kinematics of individuals with chronic ankle instability while walking and jogging on a treadmill in shoes. Phys Ther Sport 2013;14(4):232-239.
- Drewes LK, McKeon PO, Casey Kerrigan D, Hertel J. Dorsiflexion deficit during jogging with chronic ankle instability. J Sci Med Sport 2009;12(6):685-687.
- Gigi R, Haim A, Luger E, et al. Deviations in gait metrics in patients with chronic ankle instability: a case control study. J Foot Ankle Res 2015;8(1):1.
- Koldenhoven RM, Feger MA, Fraser JJ, et al. Surface electromyography and plantar pressure during walking in young adults with chronic ankle instability. Knee Surg Sports Traumatol Arthrosc 2016;24(4):1060-1070.
- Brown C. Foot clearance in walking and running in individuals with ankle instability. Am J Sports Med 2011;39(8):1769-1776.
- Konradsen L. Factors contributing to chronic ankle instability: kinesthesia and joint position sense. J Athl Train 2002;37(4):381-385.
- Neumann DA. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation. 2nd ed. St. Louis: Mosby Elsevier; 2002.
- Morrison KE, Hudson DJ, Davis IS, et al. Plantar pressure during running in subjects with chronic ankle instability. Foot Ankle Int 2010;31(11):994-1000.
- Drewes LK, McKeon PO, Paolini G, et al. Altered ankle kinematics and shank-rear-foot coupling in those with chronic ankle instability. J Sport Rehabil 2009;18(3):375-388.
- Herb CC, Hertel J. Shank-rearfoot joint coupling in young adults with chronic ankle instability: a cross-correlation analysis. J Sports Med Phys Fitness 2015;55(6):639-646.
- Donovan L, Feger MA, Hart JM, et al. Effects of an auditory biofeedback device on plantar pressure in patients with chronic ankle instability. Gait Posture 2016;44:29-36.
- Donovan L, Hart JM, Hertel J. Effects of 2 ankle destabilization devices on electromyography measures during functional exercises in individuals with chronic ankle instability. J Orthop Sports Phys Ther 2015;45(3):220-232.
- Chinn L, Dicharry J, Hart JM, et al. Gait kinematics after taping in participants with chronic ankle instability. J Athl Train 2014;49(3):322-330.
- Barlow G, Donovan L, Hart JM, Hertel J. Effect of lace-up ankle braces on electromyography measures during walking in adults with chronic ankle instability. Phys Ther Sport 2015;16(1):16-21.
- Herb CC, Chinn L, Hertel J. Altering shank-rear-foot joint coupling during gait with ankle taping in patients with chronic ankle instability and healthy controls. J Sport Rehabil 2016;25(1):13-22.
- McKeon PO, Paolini G, Ingersoll CD, et al. Effects of balance training on gait parameters in patients with chronic ankle instability: a randomized controlled trial. Clin Rehabil 2009;23(7):609-621.








