
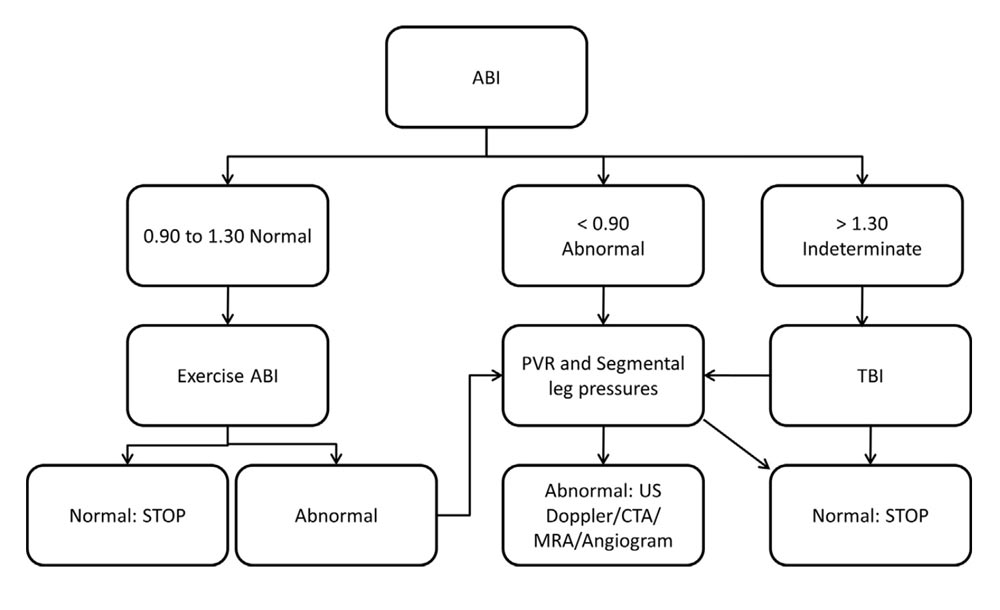
Figure 1. Algorithm for the noninvasive vascular laboratory workup of patients with suspected PAD. Please note that because of variations in physician preferences, it is common to perform rest ABI,TBI, segmental pressure measurements, and PVR in all patients with suspected PAD. ABI, ankle brachial index; CTA, computed tomography angiography; MRA, magnetic resonance angiography; PAD, peripheral arterial disease; PVR, pulse-volume recording; TBI, toe brachial index; US, ultrasound. Reprinted from McCann TE, Scoutt LM, Gunabushanam G. A practical approach to interpreting lower extremity noninvasive physiologic studies. Radiol Clin North Am. 2014;52(6), 1343–1357. Copyright © 2014 by the authors; permission for reuse provided by the lead author. Published by Elsevier Inc.
Peripheral arterial disease may be a common finding among those over 50, but it remains underdiagnosed. Choosing the right diagnostic test is key.
By Brittany Mammano, DPM, PGY-1, and Saba Sadra, DPM, MSc

Eight million men and women in the United States have lower extremity peripheral arterial disease (PAD).1 PAD is a common finding among patients over age 50, yet it is frequently underdiagnosed. An understanding of the risk factors, diagnostic techniques and treatment options is essential for proper screening and care of affected individuals. Because even asymptomatic individuals with PAD have an increased relative risk of death, screening of the at-risk population should be considered to identify the disease and begin treatment (see Figure 1). Early intervention with lipid-lowering therapy and antiplatelet drugs may delay disease progression and prevent premature death from cardiovascular causes.2
This article will review general principles, indications, interpretations, and limitations of several types of noninvasive vascular testing for peripheral arterial disease in the lower extremity.
Ultrasound
A standard vascular testing workup often includes both duplex ultrasound and physiologic evaluation. Physiologic testing or pulse-volume recording (PVR), which will be discussed in a later section of this review, evaluates the physiology of blood flow by evaluating flow pressures and waveforms. According to Gerhard-Herman et al, this includes both segmental pressures and PVR testing.3 Duplex scanning refers to an ultrasound scanning procedure recording both gray scale and Doppler information. This includes 2-dimensional structure and motion, Doppler spectrum analysis, and color flow velocity mapping.
Characteristic duplex ultrasound features of stenosis include elevated velocities, color disturbance, spectral broadening, and post-stenotic waveforms.3 Duplex ultrasound images the entire arterial tree of the lower extremity, distinguishing between occluded, stenotic, non-stenotic, and aneurysmal segments.4 Additionally, ultrasound testing with color and pulsed wave Doppler has a high sensitivity (88%) and specificity (97%) in identifying stenoses of greater than 50% in the lower extremity.3 Ultrasound has the advantage of being inexpensive, widely available, and does not use contrast media or ionizing radiation; however, it may be limited in individuals with a large body habitus, lymphedema, lower extremity wounds, and those with extensive arteriosclerotic calcifications.2,5
 Ankle-Brachial Index (ABI)
Ankle-Brachial Index (ABI)
ABI is the easiest and most widely used measurement of noninvasive arterial testing.6 It is considered a first-line test by the American Heart Association and is recommended for new patients with suspected PAD.2,5 Given the high incidence of PAD, many public health organizations are advocating screening in all patients who have exertional leg symptoms, patients age 50 – 69 with cardiovascular risk factors, and all patients older than age 70.7
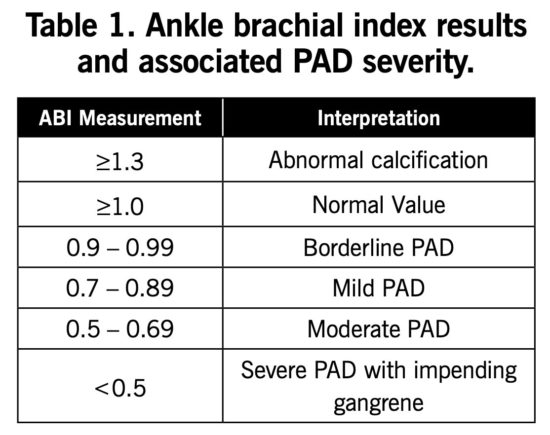
Normal pulse wave reflection in healthy individuals causes the ankle systolic pressure to be approximately 10% higher than the brachial systolic pressure; therefore, a normal value is >1.0.1 An ABI <0.9 is diagnostic for PAD in patients with clinical symptoms of claudication or other signs of impaired blood flow, with 95% sensitivity and 100% specificity.7 An ABI result >1.3 is considered abnormal due to likely vessel calcification. Further details of ABI results and interpretations are listed in Table 1.
Given that many patients with PAD are underdiagnosed and the widespread use of ABI as a screening test, it is important for the clinician to be aware of its limitations. Decreased ABI does not correlate well with symptomatic disease, and almost 50% of patients with an ABI <0.9 are asymptomatic. Even patients with borderline PAD have a high incidence of mobility loss. A 5-year follow up on the Walking and Leg Circulation Study found that patients with a borderline ABI result had a higher incidence of losing the ability to walk for 6 continuous minutes when compared to those with a normal ABI.1
Most vascular guidelines do not recommend ABI as a stand-alone test. Patients with diabetes or chronic renal failure often have calcified vessels, which often falsely elevates ankle pressure readings. In patients who have normal results on their ABI but are clinically suspected of having PAD, an exercise ABI test is recommended.5 This test allows the clinician to quantify the patient’s walking impairment and assess their functional disability.6 This also can provide an assessment of whether the suspected claudication-like symptoms are caused by arterial insufficiency versus musculoskeletal or neurologic pain.5
An abnormal ABI alone is not a reliable predictor of vessel disease magnitude, extent, or location. Additional noninvasive physiologic studies are recommended, including segmental arterial pressures, pulse volume recordings, and Doppler waveforms.7
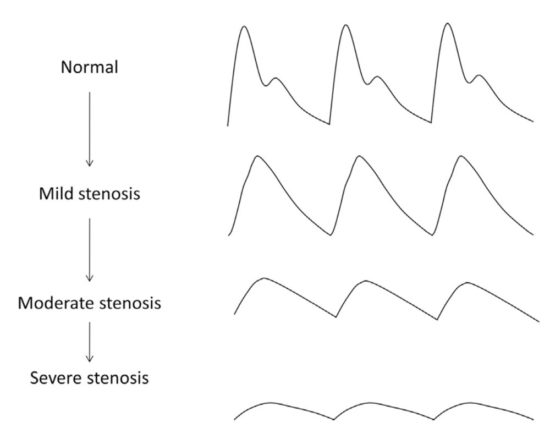
Figure 2. Schematic diagram showing the changes in pulse-volume recording waveforms with increasing severity of stenosis. Loss of the dicrotic notch is often the first observed sign. With further increase in stenosis severity, there is a progressive flattening of the waveforms, an increase in the time to peak, and the downslope becomes bowed away from the baseline. Reprinted from McCann TE, Scoutt LM, Gunabushanam G. A practical approach to interpreting lower extremity noninvasive physiologic studies. Radiol Clin North Am. 2014;52(6), 1343–1357. Copyright © 2014 by the authors; permission for reuse provided by the lead author. Published by Elsevier Inc.
Toe Brachial Index (TBI)
The toe brachial index (TBI) is similar to ABI as it is calculated by dividing blood pressure measured in the hallux or second digit by the systolic brachial blood pressure. This test is beneficial to patients as the digital arteries are much less likely to be affected by atherosclerotic calcifications of the tunica media compared with arteries of the ankle. Current international guidelines recommend TBI as an alternative screening test for PAD if ABI is elevated.7
The value of TBI is limited due to the lack of a well-established grading system to indicate the presence of disease. A normal toe pressure is listed as 80 – 90% of the brachial artery pressure; therefore, a normal TBI is 0.8 – 0.9.8 Establishing the presence of disease is not yet settled: currently, values of <0.6, <0.7, and <0.75 have been documented in the literature as a cut-off for the presence of disease.8 A toe pressure value lower than 30 mmHg or TBI <0.2 is considered severely ischemic.7 A lower TBI compared to a normal value correlates with decreased wound healing potential.
Segmental Pressure Measurements
Systolic blood pressure measurements at various points in the lower extremity can provide useful information in the assessment of PAD. A cuff is inflated to a pressure above systolic blood pressure and then slowly deflated while a Doppler device is used to detect blood flow in the distal vessels.4 In a healthy individual, blood pressures increase slightly as one moves distally within a limb. Normal pressure variation between limb segments should be no more than 20 – 30 mmHg.7 A complete arterial occlusion will be indicated by a gradient ≥ 40 mmHg.
A benefit of this type of testing is that a clinician may be able to specify an abnormal arterial segment within the lower extremity. The diagnostic accuracy of segmental pressure measurements to detect an abnormal arterial segment (between aortoiliac, femoropopliteal, and popliteal-tibial) is 87%, with a positive predictive value of 96%.9
The quality of the segmental pressure measurement is limited to technician experience, as small errors in cuff sizing will falsely elevate or lower the resulting pressure readings. Additionally, similar to the ABI limitation, arteries may be incompressible due to the presence of calcified plaque.2 Other limitations include difficulty interpreting results due to lower extremity edema and obesity.4
Pulse Volume Recording (PVR)
The PVR assessment is a pneumo-plethysmographic test used for detection of segmental volume changes. Pneumatic cuffs are placed at multiple levels along an extremity and are inflated to a standardized pressure of 65 mmHg.2 The inflation pressure allows for venous outflow occlusion and an isolated evaluation of arterial pressure waveform.4 One benefit of utilizing this modality is that PVR is not significantly affected by arterial calcifications.10 A normal pulse wave is characterized by a steep upslope, a narrow peak, the presence of a dicrotic notch in the downslope, and a concave downslope (see Figure 2).2 In the presence of proximal arterial stenosis or occlusion, usually the first observed change on PVR is loss of the dicrotic notch. As disease progresses, the amplitude of the pulse wave decreases. An amplitude of <5 mm from trough to peak has been used as a criterion for diagnosing vascular claudication.7
Research shows that PVR and ABI together improve diagnostic accuracy for PAD compared to ABI alone.10Lewis et al reported PVR with 97% sensitivity and 81% specificity.11 When PVR was combined with ABI, sensitivity for PAD went up to 100% and overall accuracy went up to 85%.
Additionally, PVR can be used to help determine acute versus chronic occlusive disease. PVR testing in combination with Doppler waveforms can help diagnose chronicity of arterial disease. In chronic occlusive disease, the body will compensate and develop collateral arterial blood supply.7 This can result in a PVR waveform that may be relatively preserved compared with the Doppler waveform. In acute thrombosis, both Doppler and PVR waveforms are absent or decreased.
There are many factors that modify and may confound accurate measurements of systolic pressure, including stroke volume, vessel wall elasticity, presence of collateral vascular beds, arteriovenous shunts, vasoconstriction or vasodilation of small arteries and arterioles, and size or positioning of the limb.2 One shortcoming of PVR testing is the subjective evaluation made by the observer: results may be less reliable with an inexperienced technician.10
Transcutaneous Oxygen Pressure (TcPO2)
Transcutaneous oxygen pressure is a noninvasive technique that can detect excess oxygen diffusion from red blood cells as they pass through capillary circulation. The measurement allows for specific analysis of oxygen delivery and consumption on regional areas before, during, and after exercise.6 This type of testing would best be used in patients suffering from clinically atypical claudication symptoms, or if other noninvasive vascular testing has been inconclusive.12 Normal TcPO2 limb values are 50 – 60 mmHg; pressures ≤20 mmHg usually are accompanied by rest pain, ischemic ulcers, or gangrene.13
TcPO2 is not affected by calcification of the tunica media, indicating its usefulness in patients with diabetes and renal disease.14 Patient positioning, an examiner’s choice in probe, and probe placement can all skew results of TcPO2 testing.6 Additionally, it can be time consuming to obtain pressure measurements with calibration, and consistent accurate results may be limited by technician experience. TcPO2 has a sensitivity of 77% at rest; however, following exercise, sensitivity has been reported to increase to 100%.13
Another use of TcPO2 testing is as adjunctive testing to hyperbaric oxygen therapy (HBOT). A prospective randomized controlled trial by Sarbjot et al found a positive correlation between TcPO2 values and various markers of wound healing. Values ≤40mmHg were associated with poor wound healing.17 This study concluded periwound TcPO2 values may be used as a predictor of response to HBOT and have a positive correlation with respect to wound healing.17
Skin Perfusion Pressures
Skin perfusion pressure (SPP) utilizes a laser Doppler and pressure cuff to assess reactive hyperemia. Normal values are >40 mmHg and considered non-ischemic. A pressure in the range of 30 – 40 mmHg is considered marginal ischemia. A pressure <30 mmHg indicates more severe ischemia, making wound healing unlikely.13 In a retrospective review of clinical reliability and utility of skin perfusion pressures in ischemic limbs compared with ABI, TBI, and TcPO2, Yamada et al found an SPP of 40 mmHg to have a 72% sensitivity and 88% specificity for wound healing.18 SPP can be used in patients with lower extremity edema, and no skin warming or other preparation is needed. No calibration is needed to complete the test, and it is faster than TcPO2.13 One shortcoming, however, is that data is only provided on the specific area of skin under the sensor; multiple separate readings are needed to have a greater perspective of a limb’s vascularity.

Figure 3. Assessment of plantar angiosome perfusion utilizing hyperspectral imaging. The visual, integrated oxyhemoglobin-deoxyhemoglobin, and only deoxyhemoglobin hyperspectral images are shown of the plantar meta- tarsal angiosome for a foot with no PAD (left) and a foot with PAD (right). The foot with PAD demonstrates substantially decreased oxyhemoglobin and deoxyhemoglobin values throughout the angiosome. Reproduced with permission from Sumpio BE, Forsythe RO, Ziegler KR, Baal JGV, Lepantalo MJ, Hinchliffe RJ. Clinical implications of the angiosome model in peripheral vascular disease. J Vasc Surg. 2013; 58(3):814–826. Copyright © 2013 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Hyperspectral Imaging
Newer technologies have been proposed in evaluating lower extremity blood flow. Hyperspectral imaging combines spectroscopy and imaging and is considered an advance of traditional near-infrared spectroscopy. Various devices are available that calculate tissue oxygen saturation from the absorption of infrared light at multiple wavelengths.6 In hyperspectral imaging the level of oxyhemoglobin (indicating oxygen delivery) is compared with deoxyhemoglobin (representing oxygen extraction). Data is then compared and a color scan is created which colorizes an image to depict the varying levels of oxygenation (see Figure 3).13 As no skin contact is required, this technology is noninvasive, is easy to use, and produces fast results. Sumpio et al reported it can detect changes in microcirculation in patients with diabetes and can predict healing of foot ulcers with a sensitivity of 86% and specificity of 88%.15 At this time, the technology is limited with few published studies validating its use in a clinical setting.
Computed Tomography Angiography (CTA) and Magnetic Resonance Angiography (MRA)
Detailed characterization of PAD can be performed with noninvasive angiography using CTA or MRA.16 Advances in both CTA and MRA provide a high resolution, 3-dimensional map of the patient’s peripheral arterial tree.
The use of MRA has grown in recent years with the development of multi-detector scanners and the ability to obtain multiple cross-sectional images with faster acquisition times. Collins et al performed a meta-analysis comparing contrast-enhanced magnetic resonance imaging (MRI), duplex sonography, and CTA and showed superior sensitivity and specificity with MRA for the detection of lesions of >50% stenosis.19 However, MRI still has some limitations such as prolonged acquisition times, limited availability of advanced MRI sequences and technology, and cost.16 Another drawback to MRA is a risk of nephrogenic systemic fibrosis which is caused by the gadolinium contrast media.5
Computed tomography angiography has excellent spatial resolution compared to duplex sonography. Several small studies of multi-detector CT scanning for PAD have shown high sensitivities (89% to 100%) and specificities (92% to 100%) for lesions with >50% stenosis.20 Limitations of CTA include issues regarding post-processing of raw data or visual limitations with artery calcifications that can lead to significant artifacts.16 Plus, CTA is relatively expensive. The use of CTA is limited in patients with renal disease, as the use of iodinated contrast media can be nephrotoxic.2
Current research continues to advance the goals in PAD screening and diagnosis with enhanced imaging by improving spatial resolution, limiting contrast use, and obtaining accurate dynamic data of blood flow.
Take Home Points:
- PAD affects a large portion of the population of the United States and early identification not only allows treatment of PAD but also modification of risk factors associated with cardiovascular disease.
- There is no clear-cut algorithm in the existing literature with a proven method for the workup of PAD with the available noninvasive studies; we provide the suggested algorithm from McCann et al2 as a framework (see Figure 1). Many institutions vary in standard testing offered in terms of available equipment and technicians. Consequently, financial cost and the time required for testing contributes to differences of care in the workup of PAD.
- Low ABI values (<0.9) along with clinical indications is positive for a diagnosis of PAD. However, a “normal” ABI result (>0.9) is unreliable in the diagnosis or in ruling out PAD, especially in diabetic patients.
- TBI may be helpful as a secondary test for PAD, but evidence for TBI as a stand-alone diagnostic test is low.
- Segmental artery pressures may be helpful for determining a segment of the lower extremity that is affected by disease. However, the test is limited by possible false elevations due to arterial calcification. Assessing results can also be difficult in patients with lower extremity obesity and edema.
- A major benefit of pulse volume recording (PVR) is that arterial calcifications are much less likely to interfere with the assessment. One of the earliest signs of PAD in PVR testing is loss of the dicrotic notch on the pulse wave.
- PVR in combination with ABI has been shown to have greater diagnostic accuracy compared with ABI alone.
- Transcutaneous oxygen pressures may be used as a predictor of response to successful wound care treatment with hyperbaric oxygen therapy.
- McDermott MM, Guralnik JM, Tian L et al. (2009). Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up. The WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53(12):1056–1062. With comment by same authors in J Vasc Surg. 2009;50(4):959.
- McCann TE, Scoutt LM, Gunabushanam G. A practical approach to interpreting lower extremity noninvasive physiologic studies. Radiol Clin North Am. 2014;52(6):1343–1357.
- Gerhard-Herman M, Gardin JM, Jaff M, Mohler E, Roman M, Naqvi TZ. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19(8), 955–972.
- Bandyk DF. Interpretation pitfalls of vascular laboratory testing. Semin Vasc Surg. 2013;26(2-3):67–71.
- Williams J, Alexander J. (2018). Noninvasive vascular diagnostic laboratory. In: Harken AH, Moore EE, ed. Abernathy’s Surgical Secrets. 7th ed. Philadelphia, PA: Elsevier, Inc. 2017:359–364.
- Abraham P, Gu Y, Guo L, et al. Clinical application of transcutaneous oxygen pressure measurements during exercise. Atherosclerosis. 2018;276:117–123.
- Sibley RC, Reis SP, Macfarlane JJ, Reddick MA, Kalva SP, Sutphin PD. Noninvasive physiologic vascular studies: a guide to diagnosing peripheral arterial disease. Radiographics. 2017;37(1):346–357.
- Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe–brachial index for detecting peripheral artery disease. Vasc Med. 2016;21(4);382–389.
- Aburahma AF, Khan S, Robinson PA. Selective use of segmental Doppler pressures and color duplex imaging in the localization of arterial occlusive disease of the lower extremity. Surgery. 1995;118(3):496–503.
- Hashimoto T, Ichihashi S, Iwakoshi S, Kichikawa K. Combination of pulse volume recording (PVR) parameters and ankle–brachial index (ABI) improves diagnostic accuracy for peripheral arterial disease compared with ABI alone. Hypertens Res. 2016;39(6):430–434.
- Lewis JE, Williams P, Davies JH. Non-invasive assessment of peripheral arterial disease: automated ankle brachial index measurement and pulse volume analysis compared to duplex scan. SAGE Open Med. 2016;4:205031211665908.
- Koch C, Chauve E, Chaudru S, Faucheur AL, Jaquinandi V, Mahé G. Exercise transcutaneous oxygen pressure measurement has good sensitivity and specificity to detect lower extremity arterial stenosis assessed by computed tomography angiography. Medicine (Baltimore). 2016;95(36):e4522.
- Shapiro J, Nouvong A. Assessment of Microcirculation and the Prediction of Healing in Diabetic Foot Ulcers. In: Zimering M, ed. Topics in the Prevention, Treatment, and Complications of Type 2 Diabetes. Rijeka, Croatia: Intech; 2011:215-226. Available at https://www.intechopen.com/books/topics-in-the-prevention-treatment-and-complications-of-type-2-diabetes/assessment-of-microcirculation-and-the-prediction-of-healing-in-diabetic-foot-ulcers. Accessed March 3, 2020.
- Hauser CJ. (1984). Superiority of transcutaneous oximetry in noninvasive vascular diagnosis in patients with diabetes. Arch Surg. 1984;119(6):690–694.
- Sumpio BE, Forsythe RO, Ziegler KR, Baal JGV, Lepantalo MJ, Hinchliffe RJ. Clinical implications of the angiosome model in peripheral vascular disease. J Vasc Surg. 2013;58(3):814–826.
- Pollak AW, Norton PT, Kramer CM. Multimodality imaging of lower extremity peripheral arterial disease: current role and future directions. Circ Cardiovasc Imaging. 2012;5(6):797–807.
- Kaur S, Pawar M, Banerjee N, Garg R. Evaluation of the efficacy of hyperbaric oxygen therapy in the management of chronic nonhealing ulcer and role of periwound transcutaneous oximetry as a predictor of wound healing response: a randomized prospective controlled trial. J Anaesthesiol Clin Pharmacol. 2012;28(1):70–75.
- Yamada T, Ohta T, Ishibashi H, et al. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47(2):318–323.
- Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
- Mathew RC, Kramer CM. Recent advances in magnetic resonance imaging for peripheral artery disease. Vasc Med. 2018;23(2):143–152.







Love your info. Just got pad test with left foot at 0.54 significant and right foot at0.89 moderate just trying to find out the signification and potential treatment of these diagnosed any comment you might have would be appreciated