Opportunistic microorganisms called biofilms constitute an age-old diabetic wound care problem that defies traditional antimicrobial therapies. Experts believe interventions customized to individual patients based on molecular diagnostics may be the best line of defense.
By Larry Hand
Everything in medicine always comes down to what’s best for an individual patient. That’s not exactly news to clinicians, but that old thought may be taking on new meaning when it comes to treating recalcitrant diabetic foot ulcers. With opportunistic microorganisms called biofilms adhering to wound beds and apparently inhibiting the healing of many chronic wounds, getting at the biofilms in their myriad configurations of bacteria and polymicrobial communities calls for a personal diagnosis and a personal treatment plan. Just perhaps, some clinicians say, the time is right to start applying personalized medicine to treat a problem about which, until recently, you could only say the patient just has to live with it.
Although researched extensively in other fields, momentum for biomedical biofilm study has picked up greatly in the past several years, after proclamations such as one during a National Institutes of Health advisory committee meeting that biofilms are present in almost 80% of infections. And the technology for studying biofilms has begun to catch up with the interest.
“By targeting the wound biofilm with powerful diagnostic tools and personalized wound care, wound healing outcomes are significantly improved. This improvement in wound healing outcomes by specifically addressing biofilm demonstrates that biofilm is indeed a right target,” wrote Randall D. Wolcott, MD, CWS, of the Southwest Regional Wound Care Center in Lubbock, TX, in a 2011 article in Plastic and Reconstructive Surgery.1
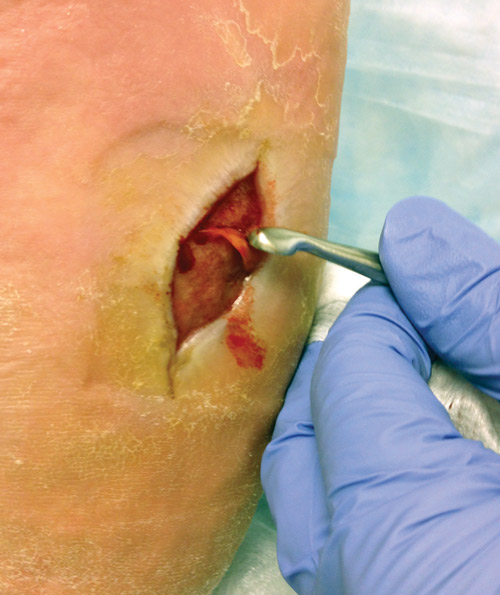
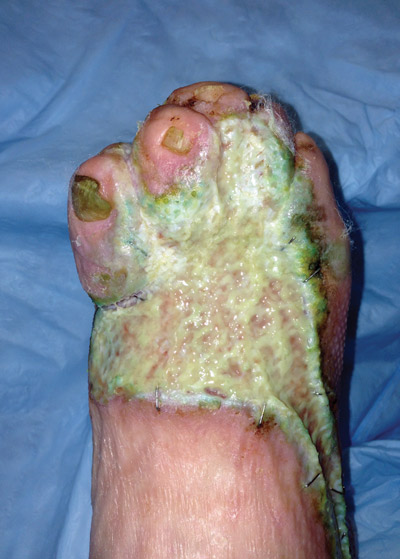
Figure 1. Plantar midfoot wound with evident thick biofilm layer on debridement. (Photo courtesy of John Steinberg, DPM.)
“It’s huge, and it’s going to be bigger than what we can talk about because you can’t get into conjecture,” Wolcott told LER in an interview. However, he added, “In webinars people are billing this as the way to heal wounds. It’s just one leg of the stool. It’s important. I think it speeds up healing and does other stuff, but you still have to do good wound care.”
John Steinberg, DPM, an associate professor in the Department of Plastic Surgery at Georgetown University School of Medicine in Washington, DC, says his practice has begun to see some positive results.
“Everybody’s talking about biofilms, but we haven’t seen a lot of action in the ability to intervene. However, I do think we’re on the cusp, if not already having turned the corner,” said Steinberg, coauthor of a 2012 review on the impact of biofilms.2
“We have a couple of key new tools now that have helped us move from talking about and kind of understanding biofilms to actually doing something about it. We’ve got the PCR [polymerase chain reaction]-based culturing that can tell us what bacteria are on the surface of the wound and what the quantity is and what the proportions are,” he said. “The other [change] is that our topicals have improved and we have now the ability to combat biofilms with different topical agents. Once we have the PCR cultures, we sometimes will also do a customized gel that is directed at those actual pathogens. It has been very effective on some of the chronic multiyear wounds.”
Ecosystems and foot ulcers
The problem with biofilms has been multilevel—first, just understanding them, and then learning how to diagnose them and target them therapeutically. Rather than being planktonic in nature as monospecies bacterium infections are in acute foot ulcers, biofilms are multispecies communities of microorganisms that adhere to the wound, colonize it, and then grow in their own ecosystem. They can contain the same bacterium that could be present in an acute wound, but along with more—sometimes many more—of the pathogens. In addition to bacteria, which make up about 20% of a biofilm’s elements, the remaining 80% include extracellular polymeric substances such as polysaccharides, proteins, and nucleic acids.3

Figure 2. Dorsal foot wound status post grafting with pseudomonas infection and thick biofilm destruction of the graft. (Photo courtesy of John Steinberg, DPM.)
“From the scientific perspective, we’re both trying to understand what’s going on on the host side with the patient, why their immune system can’t clear what we call opportunistic pathogens that are common to people’s skin but, in the case of the chronic wound, have become an infectious agent, and then understanding why the immune system isn’t responding appropriately,” Mary Cloud B. Ammons, PhD, an assistant research professor in the Department of Chemistry and Biochemistry at Montana State University in Bozeman, told LER. “On the other side, we’re trying to determine the characteristics of these opportunistic pathogens when they become the biofilm phenotype and what insight we might gain by understanding that phenotype for therapeutic purposes.”
Although biofilms take on a variety of lives themselves, some common characteristics have become apparent. In his paper, “The role of biofilms: are we hitting the right target?”1 Wolcott described those characteristics as:
- Adhering to the wound bed.
- Secreting protective substances to fight white blood cells, antibodies, and therapeutic antibiotics.
- Having different physiology and growth states for each bacterium that are guided by “quorum sensing.” Unique to biofilm, quorum sensing drives gene expression of the varying rates of growth.
- Reconstituting from leftover particles after an intervention. However, a biofilm is more vulnerable at this state to both host immunity and to treatments, “thus creating a therapeutic window.”
“Bacteria create a matrix in which they communicate, grow and colonize into a biofilm,” said David G. Armstrong, DPM, MD, PhD, professor of surgery and director of the Southern Arizona Limb Salvage Alliance in the University of Arizona School of Medicine in Tucson. “When there’s a certain quorum, the bugs start to go roaming for another place to call home, and that may be something that can be fixed. An action might be able to be taken to stop further progression.”
Back to personalized medicine
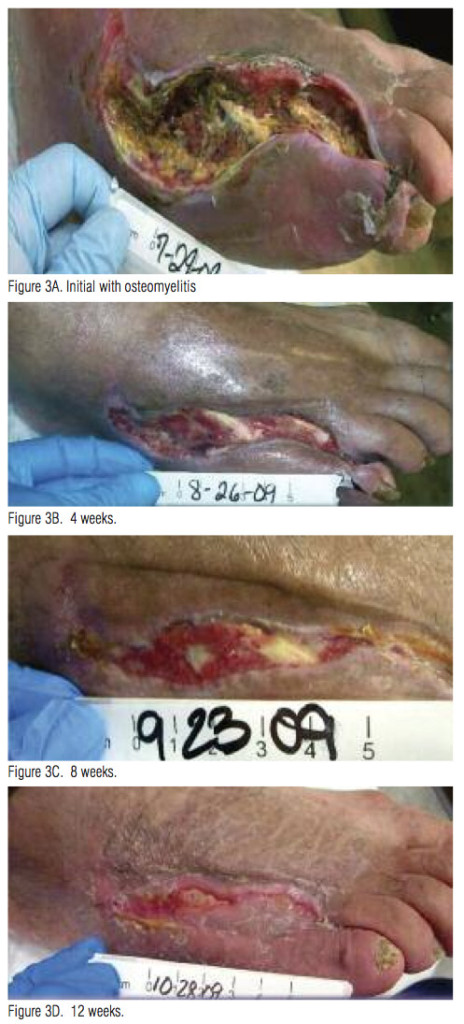 Wolcott wrote that a “diabetic foot ulcer is an excellent example of a chronic wound that responds well to management using biofilm principles.”
Wolcott wrote that a “diabetic foot ulcer is an excellent example of a chronic wound that responds well to management using biofilm principles.”
First, the wound surface must be debrided to physically disrupt the biofilm matrix. Then molecular diagnostics can be used to identify the microorganisms in the biofilm. At that point, topicals and systemic therapies can be aimed at those specific microorganisms during their time of vulnerability as they try to recolonize and form a union.
Biofilms are very different from planktonic bacteria, and they can’t be treated the same way as bacteria in an acute wound, Wolcott said.
“Their ecology, their nutritional sources, everything about them is quite different,” he said. “Systemic antibiotics can help you with your wound, but they’re not going to eradicate the biofilm. It would be like taking antibiotics to cure cavities.”
In addition to the PCR-based culturing that is available now, more diagnostics are in the pipeline, Wolcott said. Those include advanced methods of sequencing4,5 and new quantitative PCR methods.6
“Some tools will be available commercially in the coming years that will likely allow us to identify when the bugs in the biofilm, shall we say, have nefarious intent, or they’re leaving the biofilm to go wandering through the human matrix. They have to release enzymes that we can likely detect,” Armstrong said. “Those are things that might be able to be done at the bedside, and that may help to direct more active, more extensive therapies when they’re needed—or, more importantly, it may help avoid using other expensive therapies that might be wasted.”
For Crystal Holmes, DPM, CWS, assistant professor of internal medicine at the University of Michigan Health System in Ann Arbor, who specializes in treating diabetic foot ulcers, what’s missing in the clinic right now is a handheld instrument that can diagnose a biofilm at the bedside or chairside and mine a database to recommend a treatment path for the right patient at the right time.
“If I’m in a clinic with a patient, and the patient has a wound, what do I have to measure to tell me or give me an indicator of what would be the best next step for this patient at this point in time?” Holmes asked. “We have some promising things out there right now, but that’s really what we’re missing clinically.”
Holmes and her colleagues found a need for further biofilm research when they conducted a systematic review of studies reporting on using collagen wound dressings to treat diabetic foot ulcers.7 They wrote that their analysis results “suggest that future works focus on biofilms and extracellular regulation, and include high-risk patients.”
“[Biofilms are] intriguing from a research perspective, but to actually use it every day in how we treat patients, it probably isn’t quite there yet,” said her coauthor on that paper, James S. Wrobel, DPM, an associate professor of medicine in the University of Michigan Health System. “I think we’re suspicious of it when we see these wounds come in and they’re not progressing with all the other treatments we’ve tried. It looks like the dynamics of the biofilm may be implicated in the high rates of clinically infected wounds seen in large cohort studies, as well as possibly increasing bacterial virulence.”
In addition to custom gels that can be created like the ones at Georgetown, some other compounds have shown some promise in testing, including a compound of lactoferrin and xylitol, which scientists in Ammons’ lab have studied extensively,8-11 and for which Wolcott owns the patent.
“Lactoferrin is something our bodies actually produce to protect us from opportunistic pathogens,” Ammons said. “It’s in milk, but it’s also in just about all body secretions, but it’s at the highest concentrations in milk. It’s also stored in the little storage vesicles inside innate immune cells. When they run into a pathogen, they let out all these antimicrobial peptides, and lactoferrin is one of them. We’ve seen good efficacy [against biofilms] using lactoferrin, particularly in combination with xylitol, which is sugar alcohol that is found in nature, and also in combination with silver.”
Research is building
Wolcott and colleagues conducted a retrospective cohort study to compare healing outcomes for five types of wounds, including diabetic foot ulcers and venous leg ulcers, in 1378 patients in three treatment groups recruited in 2007; the findings were published in 2011.6 One group received standard treatment plus commercially available antibiofilm agents, including lactoferrin and xylitol, and traditional antibiotics chosen through empiric and culture-based methods. The second group received systemic therapy and therapy from commercially available antibiofilm agents based on the results of molecular diagnostics. The third group received personalized topical therapies (including antibiotics) after molecular diagnostics.
In the standard care group, 48.5% of patients healed completely during the seven-month study period. However, 62.4% of the patients in group two, and 90.4% of the patients in group three healed completely during that time.
“Implementation of personalized topical therapeutics guided by molecular diagnosis resulted in statistically and clinically significant improvements in outcome. The integration of molecular diagnostics and personalized medicine provides a directed and targeted approach to wound care,” the researchers wrote.
In the clinic, Wolcott says, it makes sense to base treatment strategies on a worst-case scenario, particularly with respect to
debridement.
“If you know there are these two types of infections and you know they can exist and they can morph one to the other, you have to treat for the worst,” he said. “Physically breaking up the biofilm could possibly just be scrubbing with a gauze. It’s much more
efficient to use steel, but some people under their [medical] license can’t debride. If you can some way break up the wound and force it to reconstitute itself, then the topicals will be much more effective.”
Steinberg at Georgetown agrees, at least on what tools are available now.
“It’s helped us in some of the most challenging wounds. We’ve healed wounds that otherwise we were stuck with just having to give palliative care,” he said. “Nothing is absolute, but if we identify a wound as biofilm-challenged, we finally can offer them something rather than just having a conversation and say, ‘Well, you’re just going to have to live with this for the rest of your life.’ That’s something we were not able to do just a couple of years ago.”
Still, treating wounds with biofilm-based approaches isn’t going to put any clinicians out of business.
“It is not true that if you kill a biofilm every wound heals,” Wolcott said. “An impaired host—and in a patient with a diabetic foot there are multiple impairments—allows the biofilm to get established. Suppressing the biofilm doesn’t fix all the host defects. You still have to revascularize if there’s limb ischemia, for example. You still have to do your stuff.”
Larry Hand is a writer in Massachusetts.
1. Wolcott RW, Dowd S. The role of biofilms: are we hitting the right target? Plast Reconstr Surg 2011;127(Suppl 1):28S-35S.
2. Kim PJ, Steinberg JS. Wound care: biofilm and it impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin Vasc Surg 2012;25(2):70-74.
3. Ammons MCB. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat Antiinfect Drug Discov 2010;5(1):10-17.
4. Dowd SE, Sun Y, Rhoads DD, et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 2008;8:43.
5. Dowd SE, Wolcott RD, Sun Y, et al. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 2008;3(10):e3326.
6. Dowd SE, Wolcott RD, Kennedy J, et al. Molecular diagnostics and personalised medicine in wound care: assessment of outcomes. J Wound Care 2011;20(5):232, 234-239.
7. Holmes C, Wrobel JS, MacEachern MP, et al. Collagen-based wound dressing for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes 2013;6:17-29.
8. Ammons MCB, Ward LS, Dowd S, James GA. Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferring iron chelation. Int J Antimicrob Agents 2011;37(4):316-323.
9. Ammons MCB, Ward LS, Fisher ST, et al. In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol. Int J Antimicrob Agents 2008;33(3):230-236.
10. Ammons MCB, Ward LS, James GA. Anti-biofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings. Int Wound J 2011;8(3):268-273.
11. Ammons MCB, Copié V. Mini-review: Lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling 2013;29(4):443-455.









