 By Pedro Rodrigues, MS, PT, PhD, Trampas TenBroek, PhD, and Joseph Hamill, PhD
By Pedro Rodrigues, MS, PT, PhD, Trampas TenBroek, PhD, and Joseph Hamill, PhD
Anterior knee pain is one of the most common injuries affecting runners, accounting for 25% of all running injuries.1 The etiology of anterior knee pain is multifactorial in nature,2 but one of the most commonly cited biomechanical risk factors is excessive rearfoot pronation. In this biomechanical injury paradigm, excessive rearfoot pronation is thought to induce excessive tibial internal rotation, which in turn disrupts the screw-home mechanism of the tibiofemoral component of the knee joint.3-5 However, the lower extremity is believed to compensate for this increase in tibial internal rotation by also increasing the amount of femoral internal rotation, preserving the screw-home mechanism. While these compensations are thought to maintain the normal mechanics of the tibiofemoral joint, they are believed to negatively alter those of the patellofemoral joint and, through repetition, cause injury.3,4 This biomechanical sequence of events has been the rationale of the widely accepted link between pronation and injury as well as the foundation for many treatment strategies.
Clinical orthotic studies have supported this injury paradigm. When focusing on short-term clinical outcomes, studies have shown consistently that foot orthoses reduce pain and improve the function of approximately 70% of individuals experiencing anterior knee pain.6-9 The same, however, cannot be said of biomechanical and epidemiological studies, which have consistently failed to provide evidence of a link between pronation and anterior knee pain.10-17 Although the cause of this discrepancy is effectively unknown, we believe one reason may be that excessive pronation is not being evaluated in the correct context.
Pronation is typically defined as the frontal plane angle formed between the tibia and calcaneus. The dynamic peak pronation angle (i.e., while walking or running) is then used to classify a runner as having normal or excessive pronation. Clinically, the dynamic peak pronation angle that separates normal pronation from excessive pronation is based on a practitioner’s experience and judgment. Similarly, biomechanical studies create a threshold value by using the means and standard deviations reported in the literature. However, this technique is limited in that it evaluates pronation without considering an individual’s joint anatomy, flexibility, or proximal response to pronation, and therefore is not patient specific.
In other words, should a healthy runner exhibiting 18° of dynamic peak pronation be considered an excessive pronator and at risk of injury? Conventional wisdom would say yes. However, what if this runner has 25° of pronation range of motion (ROM) available? In this scenario, despite having 18° of dynamic peak pronation, the joint still has 7° of additional ROM available. Using this frame of reference, should a clinician still consider this runner an excessive pronator? And should a runner with only 5° of dynamic peak pronation be classified as having normal pronation if she has only 6° of passive pronation ROM available, and therefore is coming within 1° of her available ROM? Using this conceptual framework, we feel that there may be a more patient-specific and optimal way of defining excessive pronation.
Pronation buffer
We recently tested the concept of evaluating pronation in the context of a joint’s available passive pronation ROM in 17 runners with anterior knee pain and 19 without.18 We first measured runners’ available passive pronation ROM using a custom-built device and a motion-capture system (Figure 1). Participants’ peak dynamic pronation when running was then captured using the same motion-capture system. We then calculated a pronation buffer from these two measurements to represent the angular distance a runner maintained from his or her end-range (pronation buffer = passive pronation ROM – peak dynamic pronation).

Figure 1. A ROM device was utilized to apply a 100-N compressive force and to passively pronate the rearfoot with a 10-Nm torque. The position of the rearfoot relative to the leg was then captured using an eight-camera motion-capture system.
Using this technique, we found that runners with anterior knee pain came significantly closer to their passive pronation ROM boundary and maintained, on average, only a 4.2° pronation buffer, while asymptomatic runners had a greater pronation buffer of 7.3° (Figure 2). This difference came from injured runners having, on average, 3° less passive pronation ROM as measured with the custom-built device. However, similar to previous research, we found no differences in peak dynamic pronation between asymptomatic and injured runners (injured = 7.7°, asymptomatic = 7.57°). Nevertheless, it is important to note that, on an individual level, this was not always the case. There were several runners who had small pronation buffers as a result of having the classic excessive dynamic peak pronation and not limited passive pronation ROM. In summary, this research supports the concept that pronation needs to be evaluated in a patient-specific and more anatomically relevant context.
A small pronation buffer could increase a runner’s risk of injury at the foot and knee for a variety of reasons. First, a small pronation buffer limits the foot’s ability to absorb or adapt to environmental perturbations from the running surface, such as cambered or rough terrain. As a result, more proximal joints such as the knee may be required to compensate and move unnaturally, increasing their risk of injury. Secondly, as a result of both the compensations and functioning near end-range, increased stress and strain could be placed on the joints’ surrounding tissue. Lastly, by limiting the rearfoot’s ability to absorb impact forces through joint rotation, greater demands again may be placed on more proximal joints. While the potential effect of a small pronation buffer on injury requires further investigation, we believe this concept could be clinically valuable in the interim.
Clinically, the pronation buffer can be calculated by evaluating a patient’s dynamic peak pronation relative to their measured passive pronation ROM. Through this procedure, a clinician can determine three things: first, whether a patient has a small pronation buffer; second, whether the small buffer is a result of large amounts of dynamic peak pronation or a lack of passive pronation ROM; and third, through the end feel, the type of tissue (bony, ligamentous, or muscular) that is limiting the rearfoot’s passive pronation ROM. Clinicians can then use this information as part of comprehensive clinical examination to direct a treatment approach.
Tailoring treatment
In cases in which a patient presents with a small pronation buffer as a result of having excessive dynamic peak pronation but a normal amount of passive pronation ROM, treatment should focus on controlling pronation. Ideally, this would be accomplished by strengthening the musculature of the lower extremity and foot,19-21 but sufficient strength gains would require a certain amount of time to accrue and individuals will likely want to continue participating in their chosen activity during this period. Therefore, we feel motion-controlling devices such as foot orthoses and stability footwear should be used as to tools to control foot motion while the strength of the foot is developed. However, as the foot strength increases, we believe these motion-controlling devices should be weaned systematically and as tolerated by the patient to continue developing the foot’s strength.
The findings from our research suggest that patients will commonly have small pronation buffers as a result of having limited passive pronation ROM. In these cases, the type of tissue-limiting passive pronation ROM should be noted, as it will help direct a clinician’s treatment strategy. For example, if there is a hard end feel into pronation and the limited motion is thought to be due to the joint’s osseous (bony) geometry, then the chance of increasing the passive pronation ROM is unlikely. In such cases a clinician should utilize motion-controlling devices such as foot orthoses or stability footwear to resist the motion of the joint as it approaches end range;22 these devices, however, should still allow enough motion for the foot to adapt to environmental perturbations. In addition, these runners should seek out relatively smooth and flat terrain to limit the motion required for the foot to adapt to running surface variations. Together, motion-controlling devices and selecting appropriate running terrain should limit dynamic peak pronation and, in turn, increase the pronation buffer a runner is working with.
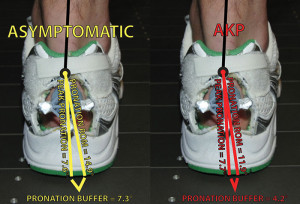
Figure 2. The pronation buffer of asymptomatic runners (yellow) and runners with anterior knee pain (red). We calculated the pronation buffer by subtracting an indi- vidual’s passive pronation ROM from his or her peak dynamic pronation value while running.
When limited passive pronation ROM is the result of a soft tissue limitation, a clinician should still consider using motion-controlling devices and limiting the running terrain during the early stages of rehabilitation. However, it is our belief that treatment should focus on increasing the joint’s passive pronation ROM through stretching or joint mobilizations.23 Additionally, concurrent focus should be placed on improving the strength and coordination of the musculature of the foot so it is prepared to control motion in this new range. Ultimately, as an individual’s passive pronation ROM increases and they are able to maintain their pronation buffer, a clinician should again start to systematically wean these individuals from motion-controlling devices.
In conclusion, we feel that evaluating excessive pronation in a patient-specific and anatomically based context, for example, determining the size and structural nature of the pronation buffer, may identify those at risk of injury more effectively than the more traditional definition of excessive pronation. In addition, we believe evaluating pronation in this context in conjunction with a thorough clinical exam will inform clinicians about the root causes of a small pronation buffer and help identify specific treatment strategies.
This novel way of evaluating pronation shows promise, but more work is necessary to continue understanding the effects of a small pronation buffer. However, in the interim, this evaluation technique may be clinically valuable.
Pedro Rodrigues, MS, PT, PhD, and Trampas TenBroek, PhD, are researchers in the New Balance Sports Research Lab in Lawrence, MA. Joseph Hamill, PhD, is professor of biomechanics in the Department of Kinesiology at the University of Massachusetts Amherst.
1. Macintyre JG, Taunton JE, Clement DB, et al. Running injuries: a clinical study of 4173 cases. Clin J Sports Med 1991;1(2):87-88.
2. Fulkerson JP, Arendt EA. Anterior knee pain in females. Clin Orthop Relat Res 2000;372:69-73.
3. Tiberio D. The effect of excessive subtalar joint pronation on patellofemoral mechanics: a theoretical model. J Orthop Sports Phys Ther 1987;9(4):160-165.
4. Buchbinder MR, Napora NJ, Biggs EW. The relationship of abnormal pronation to chondromalacia of the patella in distance runners. J Am Podiatr Med Assoc 1979;69(2):159-162.
5. Hintermann B, Nigg BM, Sommer C, Cole GK. Transfer of movement between calcaneus and tibia in vitro. Clin Biomech 1994;9(6):349-355.
6. Collins N, Crossley K, Beller E, et al. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. BMJ 2008;337:a1735.
7. Amell TK, Stothart JP, Kumar S. The effectiveness of functional foot orthoses as a treatment for patellofemoral stress syndrome: the client’s perspective. Physiother Can 2000;52(2):153-157.
8. Saxena A, Haddad J. The effect of foot orthoses on patellofemoral pain syndrome. J Am Podiatr Med Assoc 2003;93(4):264-271.
9. Barton CJ, Menz HB, Crossley KM. The immediate effects of foot orthoses on functional performance in individuals with patellofemoral pain syndrome. Br J Sports Med 2010;45(3):193-197.
10. Powers CM, Chen PY, Reischl SF, Perry J. Comparison of foot pronation and lower extremity rotation in persons with and without patellofemoral pain. Foot Ankle Int 2002;23(7):634-640.
11. Hetsroni I, Finestone A, Milgrom C, et al. A prospective biomechanical study of the association between foot pronation and the incidence of anterior knee pain among military recruits. J Bone Joint Surg Br 2006;88(7):905-908.
12. Kaufman KR, Brodine SK, Shaffer RA, et al. The effect of foot structure and range of motion on musculoskeletal overuse injuries. Am J Sports Med 1999;27(5):585-593.
13. Lun V, Meeuwisse WH, Stergiou P, Stefanyshyn D. Relation between running injury and static lower limb alignment in recreational runners. Br J Sports Med 2004;38(5):576-580.
14. Messier SP, Davis SE, Curl WW, et al. Etiologic factors associated with patellofemoral pain in runners. Med Sci Sports Exerc 1991;23(9):1008-1015.
15. Walter SD, Hart LE, McIntosh JM, Sutton JR. The Ontario cohort study of running-related injuries. Arch Int Med 1989;149(11):2561-2564.
16. Duffey MJ, Martin DF, Cannon DW, et al. Etiologic factors associated with anterior knee pain in distance runners. Med Sci Sports Exerc 2000;32(11):1825-1832.
17. Levinger P, Gilleard W. Tibia and rearfoot motion and ground reaction forces in subjects with patellofemoral pain syndrome during walking. Gait Posture 2007;25(1):2-8.
18. Rodrigues PA, TenBroek T, Hamill J. Runners with anterior knee pain utilize a greater percentage of their available pronation range of motion. J Appl Biomech 2012 Jul 6. [Epub ahead of print]
19. Headlee DL, Leonard JL, Hart JM, et al. Fatigue of the plantar intrinsic foot muscles increases navicular drop. J Electromyogr Kinesiol 2008;18(3):420-425.
20. Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech 2009;24(1):26-34.
21. Brüggemann GP, Potthast W, Braunstein B, Niehoff A. Effect of increased mechanical stimuli on foot muscles functional capacity. Presented at International Society of Biomechanics 20th Congress—American Society of Biomechanics 29th Annual Meeting, Cleveland, OH, August 2005.
22. Rodrigues PA, Chang R, TenBroek T, Hamill J. Medially posted insoles consistently influence foot pronation in runners with and without anterior knee pain. Gait Posture 2012 Nov 5. [Epub ahead of print]
23. Trevino SG, Buford WL, Vallurupalli S, et al. Use of a patient-controlled stretching device to improve the ankle range of motion. Foot Ankle Int 2009;30(2):110-114.









In 1978 I lectured that the cause of chondromalacia was not pronation but the knee compensation which consisted of the motion of abduction of the distal tibia combined with internal rotation of the femur.
Back then according to the Root theory the foot would pronate for a variety of reasons which would result in internal tibial rotation. This would actually decrease the Q angle. The knee compensation for the myriad of pronatory factors results in an increase in the Q angle for the myriad of pronatory factors.
I did a study that was not published taping the foot maximally supinated and measuring the Q angle which increased with the taping.
Dr. Beekman, thanks for the information above. We have begun investigating the relationship / coordination patterns between the knee and foot in those with large and small pronation buffers. I am hopeful this will give us a better understanding of exactly how/when the knee compensates. While it is still to be determined, I do believe we will see the compensations you described. Thanks again.
Great article and observations on the notion of “excessive” pronation – agree that any amount of pronation (or any joint motion mostly) needs to be “relative” – that is, relative to its potential. The idea of Pronation Buffer makes sense…and needs to be studied further – hopefully your article will direct further research and/or re-evaluation of prior research processes related to rearfoot motion and lower extremity pathomechanics. thanks!
Thanks Daniel.