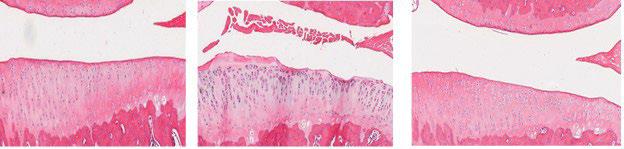
Histology of the medial tibial plateau 6 weeks after ACL transection surgery. Left: Control knee (no surgery). Middle: A knee that had an ACL transection followed by a PBS injection. Right: A knee that had an ACL transection followed by an injection of J-PRO.
A team of investigators at Boston Children’s Hospital and Rhode Island Hospital received a Military Medical Research and Development Award from the Joint Warfighter Medical Research Program (JWMRP) in 2016 to advance the development of a novel treatment for post-traumatic osteoarthritis (PTOA)—the stiffness and swelling that can result from the loss of cartilage and increased inflammation due to a joint injury. Their aim was to find another treatment option for PTOA and osteoarthritis (OA) given their prevalence: OA is the most common causes of disability in adults, and PTOA is the most common cause of disability in service members. The resulting product, J-PRO, is an injectable extracellular matrix composite that can be mixed with a patient’s blood and injected into the injured joint to reduce the risk of developing PTOA.
The investigative team, led by Martha Murray, MD, Boston Children’s Hospital, and Braden Fleming, PhD, Rhode Island Hospital, has completed preliminary efficacy studies of J-PRO in a rat anterior cruciate ligament (ACL) transection model comparing a phosphate buffered saline (PBS) (control) injection to J-PRO administration. After 6 weeks of healing, hind limbs were retrieved and assessed by histology. While the ACL transection followed by a PBS injection led to significant structural damage, this was not seen in the ACL transection followed by J-PRO injection. Severe PTOA developed in 20% of the knees that received PBS injection compared to only 5% that received J-PRO. The team is now moving forward to assess J-PRO in additional preclinical models of OA.
With the JWMRP support, the investigative team has been working with the US Food and Drug Administration (FDA) toward establishing a regulatory pathway for J-PRO. The team is compiling the necessary preclinical information including manufacturing, biocompatibility, and stability studies, to support their FDA application and ultimately receive approval for a first-in-human clinical trial of J-PRO.