 Patients with chronic refractory lower extremity tendinopathies often continue to have symptoms after exhausting most of their therapeutic options. Preliminary evidence supports the use of platelet-rich plasma in these patients, though higher-level research is needed.
Patients with chronic refractory lower extremity tendinopathies often continue to have symptoms after exhausting most of their therapeutic options. Preliminary evidence supports the use of platelet-rich plasma in these patients, though higher-level research is needed.
By Ricardo E. Colberg, MD, and Kenneth Mautner, MD
About 40% of all sports-related injuries involve tendons.1 Patellar tendon injuries, for example, are the most common knee injury in jumping sports.2 Achilles tendon injuries occur 10 times more frequently in runners than in age-matched nonrunners.3 However, sedentary people are also at risk of developing tendon injuries.1,4 Up to one third of Achilles tendinitis cases have been reported in patients who do not perform vigorous physical activity.5
Lower extremity tendon injuries frequently progress to chronic tendinopathies. Multiple extrinsic and intrinsic factors may contribute to suboptimal healing, pain, and dysfunction. Platelet-rich plasma (PRP) injections have been shown to restore the healing process and provide significant improvement in both pain and functional scores.
Tendon function and composition
Tendons are the load-bearing attachments of muscles into bones that allow a muscle contraction to translate into mechanical movement of the skeleton. They originate at the myotendinous junction, where the tendon fibers are intertwined into deep recess between the muscle fibers, and insert into the enthesis, defined as the fibrocartilage interface between the tendon fibers and the bone.
Tendons are the strongest soft tissue structures in the human body in terms of resisting tensile strength, capable of supporting up to 12 times the body’s weight.6 This strength comes from the tendon’s structure, which is mostly made up of collagen fibers aligned parallel to each other. The maximum tensile strength that a tendon can withstand is directly proportional to the number of collagen fibers and the thickness of the tendon. The ongoing tensile stress that tendons experience on a daily basis stimulates the tenocytes, which are the main cells found in tendons, to maintain the optimal ratio of collagen fibers to noncollagenous structures in the tendon.
Pathophysiology of tendon injuries
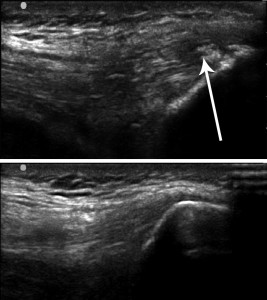
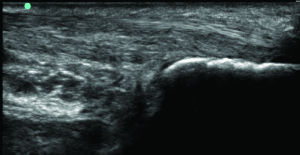
Figure 1. Intratendinous calcification at the insertion of the quadriceps tendon into the patella (above) versus a normal quadriceps tendon (below).
In order to sustain the muscle contraction necessary to withstand these high tensile forces for long periods, the tendon fibers have relatively low blood perfusion and can function at a low metabolic rate through anaerobic metabolism. In fact, some tendons, especially those with tendon sheaths, such as the extensor hallucis longus, are relatively avascular past the proximal third of the tendon closest to the muscle belly.
In acute tendon injuries, platelets that are naturally attracted to the injured site during the healing process release growth factors that stimulate formation of new blood vessels through the tendon, which in turn provide the appropriate nutrients for tenocytes to replicate and fibroblasts to lay down new collagen through the injured site. These blood vessels eventually collapse and get reabsorbed by the tendon as the newly laid collagen aligns parallel to the tendon and strengthens.
The healing process may be impaired in chronic compression injuries as well as in subacute injuries involving repetitive shear forces.7 Intrinsic factors such as age, gender, height, weight, tendon vascularity, and medical comorbidities, as well as extrinsic factors such as poor biomechanics, repetitive stress through the injured site, and changes in training patterns affect the healing process of injured tendons. If the patient does not follow a proper rehabilitation protocol that promotes tissue healing, pathologic changes occur in the tendon that are detrimental to its function. Examples of these changes are neovascularization, tissue fibrosis, collagen degeneration, intratendinous calcification, and deposition of noncollagenous substances within the tendon3,8 (Figure 1).
Platelet-rich plasma
PRP injection has been introduced as an alternative for treating chronic tendinopathies that carries minimal risk. Its use for musculoskeletal injuries (Figure 2) has increased recently, given its safety and availability for outpatient preparation and delivery.9
PRP is considered an ideal autologous blood product that promotes the body’s own natural healing.10 This concentrate has been used for more than 25 years in oral and maxillofacial surgery, otorhinolaryngology, plastic surgery, and general surgery, with multiple case series reports showing overall improvement in soft tissue healing.11-13 It has recently become popular for management of musculoskeletal injuries based on in vitro studies reporting an enhance- ment of the recruitment, proliferation, and differentiation of the cells involved in muscular tissue regeneration.13,14 Furthermore, randomized controlled trials, prospective case series, and case-control studies detailed below have shown successful clinical outcomes in treating different types of lower extremity tendinopathies.
PRP is formed by drawing blood from the patient, centrifuging the blood until the red blood cells, platelets, and plasma separate, and then extracting the platelet concentrate from the platelet-rich section of the centrifuged plasma (Figure 3). Platelets are known to carry alpha granules and dense granules, which contain multiple growth factors and cytokines that promote wound healing.12,15 Several of these growth factors have been found to improve tendon regeneration, increase tensile force, and enhance tendon healing.4,16,17 An in vivo study reported that use of PRP in human tendon injuries stimulates tenocyte proliferation, collagen production, and angiogenesis through the injured site.14
Evidence for PRP in tendon injuries
PRP has not been well studied in acute tendon injuries. Karli and Robinson reported a case of a woman aged 48 years with an acute proximal hamstring tendon partial tear who was treated with a PRP injection two weeks after the injury, followed by a rehabilitation protocol. The patient experienced significant symptom improvement and showed magnetic resonance imaging (MRI) evidence of significant tissue healing four months after the procedure.7 The authors suggested that PRP may be considered for acute partial tendon tears.
For most complete tendon ruptures, the gold standard is surgical repair. Sanchez et al investigated through a case-control study whether recovery after surgery would be accelerated by adding PRP at the injured site. They retrospectively looked at six athletes who underwent surgical repair for a complete Achilles tendon rupture and compared them to six athletes who received the same surgical repair plus a PRP injection at the injured site. The patients who received the PRP injection recovered their range of motion more quickly (seven weeks vs 11 weeks) and returned to sports participation earlier (14 weeks vs 21 weeks) than the surgery-only group and had no wound complications.18
Only one study in the literature has evaluated outcomes associated with PRP as a first-line treatment for chronic nontraumatic midportion Achilles tendinosis. This randomized double-blind placebo-controlled trial compared PRP injection and eccentric exercises versus saline injection and eccentric exercises for Achilles tendinitis that had not received previous treatment. Both injections were performed with limited fenestration of 15 passes through the tendon. The findings were reported in three separate articles.
The researchers found both groups showed significant improvement in the Victorian Institute of Sports Assessment-Achilles (VISA-A) questionnaire with no statistically significant difference between the two groups after 24 weeks.19 There was no significant difference in ultrasonographic tissue characterization between the two groups in terms of type of collagen and neovascularization seen in the tendon at 24 weeks.20 At one year follow up, the same research group once again reported no significant difference between the PRP and the saline injection.21 It is well known that most cases of nontraumatic tendinopathy will eventually heal.
In addition, both the control and the PRP groups performed eccentric exercises following the injection, which has been documented as beneficial for Achilles tendinopathy,22 and the “placebo” was actually an active treatment as needling an injured tendon has a therapeutic effect.23 Finally, De Vos et al reported that 78% of patients treated with PRP returned to sports versus 67% of patients treated with saline.19 This difference was not statistically significant.
The findings suggest PRP may have no added benefit compared with saline injection when used as first-line treatment for chronic nontraumatic Achilles tendinosis.
Most of the literature on PRP reports benefits of this technique when it is used to treat chronic tendinopathies that have failed conservative treatments. The earliest study on PRP for lower extremity tendinopathies was published by Kon et al in 2009.24 In this case-series report, 20 patients with chronic patellar tendinosis recalcitrant to conservative measures received ultrasound-guided tendon fenestration and PRP injections at the pathologic site every 15 days for a total of three procedures. Statistically significant improvement was observed for all functional and sports-specific scores and for radiographic imaging findings at six months after the last procedure.
Filardo et al then conducted a prospective case-control study comparing PRP to a physiotherapy protocol.25 The experimental group included 15 patients who had had chronic patellar tendinosis for an average of two years and had failed multiple conservative and surgical treatments; this group underwent the same PRP treatment protocol described by Kon et al. The control group consisted of 16 patients matched for sex, age and sports activity who had had patellar tendinosis for an average of eight months and had not received any prior treatment; this group underwent a physiotherapy protocol.
The PRP group demonstrated statistically superior improvement in symptoms and functional scores at six months, including the Tegner sports activity score, EuroQol Visual Analogue Scale (EQ-VAS), pain level, and patient satisfaction.25 Most recently, Filardo and Kon published a prospective case series of 43 patients with chronic proximal patella tendinopathy treated with the same PRP treatment protocol and followed for a minimum of three years.26
In this study, investigators obtained results similar to those reported above. In particular, after a mean follow up of four years the VISA-P (Victorian Institute of Sports Assessment-Patellar) score increased from 44.1 ± 15.6 at baseline to 84.3 ± 21.6 (score range is 0-100, with 100 representing an asymptomatic fully performing individual). These three studies by Kon and Filardo suggest that patients with recalcitrant cases of patellar tendinopathies treated with PRP may experience better results than patients treated with physiotherapy alone.
More recently, various studies have looked at PRP for patellar tendinopathy. Gosens compared outcomes after PRP injection for 36 patients with chronic patellar tendinopathies, 14 of whom had failed prior treatments and 22 of whom had no prior treatment.27 Both groups showed statistically significant improvement in pain as measured using a visual analog scale. On the VISA-P questionnaire, the patients who had not received prior treatment outscored the group with recalcitrant tendinopathy. This study suggests that PRP may be beneficial as a first-line treatment for chronic patellar tendinopathy.
A randomized controlled trial by Vetrano et al compared PRP injection and shock wave therapy in 46 consecutive athletes with jumper’s knee who were matched for age, sex, level of sports participation, and pretreatment clinical status.28 There was improvement in both groups, with a significantly better outcome in the PRP group at six and 12 months after treatment.
Another recent study that deserves mentioning is a case series report by Kaux and Croisier et al on 20 patients with chronic upper patellar tendinopathy who were treated with PRP.29 Patients demonstrated significant improvement in symptoms at three months, with more than 75% of patients returning to sports. The authors also reported significantly better outcomes in younger patients (24.7 years vs 32.2 years), which is promising when considering treatment options for junior and collegiate-level athletes with chronic tendinopathies.
In 2010, Gaweda et al published a case series report on 14 patients who had had chronic recalcitrant Achilles tendinopathy for an average of six months and had failed conservative treatments. Patients were treated with PRP injections and followed prospectively for 18 months.30 Outcomes were determined using the American Orthopedic Foot & Ankle Society (AOFAS) scale, the VISA-A scale, and imaging changes documented with ultrasonography. Of the 14 patients, 11 demonstrated significant improvement in the AOFAS score, VISA-A score, and sonographic imaging. Specifically, the AOFAS score improved from 55 points to 96 points, and the VISA-A score from 24 points to 96 points.
Similar findings were documented in another case series report by Monto on 29 patients with chronic recalcitrant Achilles tendinosis who had had symptoms for a minimum of six months, all of whom were all considering surgical intervention but were instead treated with a single PRP injection.31 Nearly all (27 out of 29) patients experienced significant improvement, with an average improvement in the AOFAS scale from 34 to 92 by three months, which remained elevated at 88 after two years. In addition, 27 out of 29 patients showed resolution of pathology on MRI and ultrasound imaging at six months postprocedure. Both Gaweda et al and Monto showed that PRP injections can have statistically significant benefits for the treatment of chronic recalcitrant Achilles tendinopathy. Other case series have reported similar outcomes and benefits for lower extremity tendinopathies.32-35
PRP also has been studied with regard to plantar fasciitis, which is a fibrous tissue in the foot that presents degenerative changes similar to those seen in tendinopathy. Barrett et al evaluated nine patients with recalcitrant plantar fasciopathy who were treated with ultrasound-guided PRP injections and followed for one year.36 Seven patients reported complete resolution of symptoms and had sonographic evidence of tissue improvement.
Ragab et al prospectively followed 25 patients with chronic plantar fasciitis treated with PRP injections and reported improvement in pain scores from 9.1 out of 10 prior to injection down to 1.6 out of 10 an average of 10 months after PRP, with sonographic improvement in the tissue quality and no complications.37 Similar outcomes were also reported by Martinelli et al for 14 patients treated with PRP injections for chronic plantar fasciitis.38
A case-control study by Aksahin et al compared plantar fasciitis outcomes after PRP injection or corticosteroid injection, the most common injection therapy for this condition.39 They reported visual analog scale improvement of about 50% in both groups at six months, as well as significant improvement in the modified criteria of the Roles and Maudsley scores in the PRP group. Fat pad atrophy is a possible complication of steroid injections for plantar fasciitis,40 and PRP is not associated with fat pad atrophy. If both techniques produce similar improvement in symptoms and sonographic findings, PRP may soon be considered the treatment of choice for plantar fasciopathy.
Two recent studies with large sample sizes looked at outcomes of patients with various chronic recalcitrant tendinopathies treated with PRP injection.
The first was a multicenter study by Mautner et al in which 180 patients who had had recalcitrant tendinopathy for an average of 36 months were treated and followed for an average of 15 months.41 This case series report compared outcomes of various tendinopathies of the upper and lower extremities treated with PRP. The lower extremity tendinopathies treated involved the Achilles, patella, hamstring, gluteus medius, plantar fascia, quadriceps, hip adductor, peroneus longus, tensor fascia lata, peroneus brevis, posterior tibialis, popliteus, and obturator internus tendons.
In all, 82% of patients reported moderate to complete resolution of symptoms. In addition, the average visual analog scale score for pain decreased from 7 to 1.8, 95% of patients had no pain at rest that disrupted their activities of daily living, and 85% of patients were satisfied with the procedure. The best outcome was noted in cases of Achilles tendinopathy, in which 100% of patients reported moderate to complete resolution of symptoms.
In the second study, by Harmon et al, 99 patients who had had chronic tendinopathies for an average of 34.4 months were treated with regenerative injections and prospectively followed for one year.42 The sample included 52 cases of lower extremity tendinopathies, including proximal hamstring, Achilles, patellar tendinopathy, and plantar fasciosis. Specifically, they compared autologous blood injection, leukocyte-rich PRP, and leukocyte-poor PRP. All three groups showed improvement in symptoms (70% of patients in the autologous blood group, 78% in the leukocyte-rich PRP group, and 92% in the leukocyte-poor PRP group). This study suggests that PRP is better than autologous blood injections, and leukocyte-poor PRP may be superior to leukocyte-rich PRP, though the differences in this study were not statistically significant.
Other benefits of PRP
Platelet-rich plasma injections are widely accepted by patients because the PRP is produced from the patient’s own blood and the risk of adverse effects (other than pain at the injection site) is minimal.
In a meta-analysis of studies on autologous blood products and PRP injections, no complications were reported for either procedure.43 In fact, a study on the antibacterial effect of platelet-rich gel in vitro found that the gel inhibited the growth of Staphylococcus aureus and Escherichia coli, making it a reasonable treatment option for infected wounds with delayed healing.44 This antibacterial property would theoretically reduce any infection risk associated with the PRP injection.
In addition, since PRP is extracted from the patient’s own blood, the risk of acquiring a transmitted blood-born infection or suffering an anaphylactic reaction is extremely low.10 PRP is also significantly less expensive than surgical management, which is frequently the last resort for chronic tendinopathies. Furthermore, the evidence available in the medical literature is not strong enough to reliably support surgical management of recalcitrant tendinopathies.45,46
Limitations of PRP
Platelet-rich plasma injection still has a long road ahead before it is considered the treatment of choice for chronic tendinopathies. This is because most published studies have been case series reports or case-control studies, frequently with small sample sizes, limiting the generalizability of the findings.47 Also, the side effects of PRP injections have yet to be fully defined.
A 2002 article explored the relationship between the increased concentration of growth factors in PRP and carcinogenesis.48 Based on the available evidence, the authors hypothesized that PRP could not initiate carcinogenesis but could promote it if the PRP is injected near a carcinogenic lesion. They therefore recommended avoiding PRP in patients with known cancer.
In terms of complications from PRP injection, a case report of PRP injection for recalcitrant patellar tendinopathy in a patient aged 35 years with type 1 diabetes reported an exuberant inflammatory reaction.49 In this case, the patient began submaximal eccentric exercises one week after the PRP injection.
Bowman et al reported similar complications in three patients who received PRP for patellar tendinopathy and experienced worsening symptoms after the procedure. In this article, two out of three patients did not receive formal physical therapy after the PRP treatment and the third patient started a physical therapy regime seven days after the procedure, but it is unknown when eccentric exercises were incorporated into this regimen.50
Most studies that we have reported on in this review suggest prescribing a formal physical therapy program after PRP treatment and waiting four to six weeks before starting eccentric exercises. Nonetheless, worsening of symptoms after the PRP procedure is a potential risk that should be explained to patients.
An additional question that remains unanswered is whether the trauma of the needle entering the tendon multiple times for PRP delivery is actually the main contributor to the healing of the tendon. Various studies on needle fenestration of chronic tendinopathies without corticosteroid injection have reported good to excellent outcomes.23,51,52 In addition, a single-center, prospective, randomized, double-blinded, controlled study by Rha et al found significantly better outcomes for supraspinatus tendon fenestration with PRP injection than without PRP in terms of the Shoulder Pain and Disability Index score and passive range of motion.53 A study comparing fenestration with and without PRP for lower extremity tendinopathy is warranted.
Finally, the optimal preparation and protocol for PRP has yet to be defined. Mautner et al suggested that higher platelet counts, no neutrophils, many lymphocytes, a slightly acidic pH, ultrasound guidance, and a specific rehabilitation protocol that includes eccentric exercises may be ideal to facilitate the healing of tendons following PRP injections.54 Van Ark proposed a similar rehabilitation protocol for patellar tendinopathy treated with PRP injection, and reported improvement of at least 30 points on the VISA-P scale after 26 weeks in five of six tendons.55 A consensus statement from a national or international society on the ideal PRP protocol hopefully will be published in the coming years, given the recent dramatic increase in literature published on this topic.
Conclusion
Patients with chronic refractory lower extremity tendinopathies have very few options other than innovative minimally invasive treatments such as PRP. The overwhelming majority of studies in the literature report favorable outcomes after treatment of lower extremity tendinopathies with PRP injections, whereas only a few articles have reported adverse effects or no significant added benefit of the treatment.
Certainly, the benefits of PRP seem to outweigh the potential risks. Nonetheless, there is a need for more randomized clinical trials on PRP treatment of lower extremity tendinopathy to confirm the findings. For now, the current literature supports the potential application of PRP injection for treatment of acute and chronic partial tendon tears, plantar fasciopathy, chronically painful tendons that have failed to improve despite appropriate conservative treatments, and as an adjunct treatment for surgically repaired acute tendon ruptures.
Ricardo Colberg, MD, is a physical medicine and rehabilitation physician with a subspecialty in sports medicine at Andrews Sports Medicine and Orthopaedic Center in Birmingham, AL. He is affiliated with the American Sports Medicine Institute. Kenneth Mautner, MD, is assistant professor in the departments of physical medicine and rehabilitation and orthopedic surgery at Emory University in Atlanta, GA.
Disclosures: Kenneth Mautner, MD, is on the Speaker’s Bureau for Harvest Technologies; Ricardo Colberg, MD, has no conflicts of interest.
- Järvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin 2005;10(2):255-266.
- Peers KH, Lysens RJ. Patellar tendinopathy in athletes: current diagnostic and therapeutic recommendations. Sports Med 2005;35(l):71-87.
- Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. J R Soc Med 2004;97(10):472-476.
- Kasemkijwattana C, Menetrey J, Bosch P, et al. Use of growth factors to improve muscle healing after strain injury. Clin Orthop Relat Res 2000;(370):272-285.
- Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int 1997;18(9):565-569.
- Doral MN, Alam M, Bozkurt M, et al. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):638-643.
- Karli DC, Robinson BR. Platelet rich plasma for hamstring tears. Practical Pain Manage 2010 June;10-14.
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology 2006;45(5):508-521.
- Hall MP, Band PA, Meislin RJ, et al. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg 2009;17(10):602-608.
- Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma vs corticosteroid injection with a 1-year follow up. Am J Sports Med 2010;38(2):255-262.
- Mehta S, Watson JT. Platelet-rich concentrate: basic science and clinical applications. J Orthop Trauma 2008;22(6):432-438.
- Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br 2009;91(8):987-996.
- Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009;37(11):2259-2272.
- De Mos M, van der Windt AE, Jahr H, et al. Can platelet rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 2008;36(6):1171-1178.
- Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med 2009;28(1):113-125.
- Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 2006;77(5):806-812.
- Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol 2008;215(3):837-845.
- Sánchez M, Anitua E, Azofra J, et al. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 2007;35(2):245-251.
- de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 2010;303(2):144-149.
- de Vos RJ, Weir A, Verhaar JA, et al. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med 2011;45(5):387-392.
- de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med 2011;39(8):1623-1629.
- Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med 2004;38(1):8-11.
- McShane JM, Shah VN, Nazarian LN. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow: is a corticosteroid necessary? J Ultrasound Med 2008;27(8):1137-1144.
- Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: New clinical application: A pilot study for treatment of jumper’s knee. Injury 2009;40(6):598-603.
- Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop 2010;34(6):909-915.
- Filardo G, Kon, E, Di Matteo B, et al. Platelet-rich plasma for the treatment of patellar tendinopathy: clinical and imaging findings at medium-term follow-up. Int Orthop. 2013;37(8):1583-1589.
- Gosens T, Den Oudsten BL, Fievez E, et al. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: a prospective cohort study and the influence of previous treatments. Int Orthop 2012;36(9):1941-1946.
- Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med 2013;41(4):795-803.
- Kaux JF, Croisier JL, Bruyere O, et al. Platelet-rich plasma (PRP) to treat chronic upper patellar tendinopathies. Presented at the 3rd European College of Sports and Exercise Physicians conference, Frankfurt, Germany, April 2013.
- Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med 2010;31(8):577-583.
- Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int 2012;33(5):379-385.
- Ferrero G, Fabbro E, Orlandi D, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound 2012;15(4):260-266.
- Finnoff JT, Fowler SP, Lai JK, et al. Treatment of chronic tendinopathy with ultrasound-guided needle tenotomy and platelet-rich plasma injection. PM R 2011;3(10):900-911.
- Lopez-Gavito E, Gomez Carlin LA, Parra-Tellez P, Vazquez-Escarrilla J. Platelet-rich plasma for managing calcaneus tendon tendinopathy and plantar fasciitis. Acta Ortop Mex 2011;25(6):380-385.
- Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int 2011;32(11):1032-1039.
- Barrett S, Erredge S. Growth factors for chronic plantar fasciitis. Podiatry Today 2004;17:37-42.
- Ragab EM, Othman AM. Platelet rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg 2012;132(8):1065-1070.
- Martinelli N, Marinozzi A, Carni S, et al. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop 2013;37(5):839-842.
- Aksahin E, Dogruyol D, Yuksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg 2012;132(6):781-785.
- Brinks A, Koes BW, Volkers AC, et al. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206.
- Mautner K, Colberg RE, Malanga G, et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: A multicenter, retrospective review. PM R 2013;5(3):169-175.
- Harmon K, Drezner J, Rao A. Platelet rich plasma for chronic tendinopathy. Presented at the 2nd International Scientific Tendinopathy Symposium, Vancouver, BC, September 2012.
- de Vos RJ, van Veldhoven PL, Moen MH, et al. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull 2010;95:63-77.
- Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 2007;89(3):417-420.
- Coleman BD, Khan KM, Maffulli N, et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports 2000;10(1):2-11.
- Larsson ME, Käll I, Nilsson-Helander K. Treatment of patellar tendinopathy–a systematic review of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1632-1646.
- Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R 2011;3(3):226-250.
- Martinez-Gonzalez JM, Cano-Sanchez J, Gonzalo-Lafuente JC, et al. Do ambulatory-use platelet-rich plasma (PRP) concentrates present risks? Med Oral 2002;7(5):375-390.
- Kaux JF, Croisier JL, Leonard P, et al. Exuberant inflammatory reaction after an infiltration of platelet-rich plasma (PRP). Presented at the 3rd European College of Sports and Exercise Physicians conference, Frankfurt, Germany, April 2013.
- Bowman KF Jr, Muller B, Middleton K, et al. Progression of patellar tendinitis following treatment with platelet-rich plasma: case reports. Knee Surg Sports Traumatol Arthrosc 2013;21(9):2035-2039.
- McShane, JM, Nazarian, LN, Harwood, MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med 2006;25(10):1281-1289.
- Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med 2009;28(9):1187-1192.
- Rha DW, Park GY, Kim YK, et al. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil 2013;27(2):113-122.
- Mautner K, Malanga G, Colberg R. Optimization of ingredients, procedures, and rehabilitation for platelet rich plasma injections for recalcitrant tendinopathy. Pain Manage 2011;1(6):523-532.
- van Ark M, van den Akker-Scheek I, Meijer LT, Zwerver J. An exercise-based physical therapy program for patients with patellar tendinopathy after platelet-rich plasma injection. Phys Ther Sport 2013;14(2):124-130.