 Researchers have established that elevated levels of glycated hemoglobin in patients with diabetes are associated with poor outcomes after foot and ankle surgery. Now the challenge is to identify an “acceptable” glycated hemoglobin level below which the benefits of surgery outweigh the risk of complications.
Researchers have established that elevated levels of glycated hemoglobin in patients with diabetes are associated with poor outcomes after foot and ankle surgery. Now the challenge is to identify an “acceptable” glycated hemoglobin level below which the benefits of surgery outweigh the risk of complications.
By Naohiro Shibuya, DPM, MS, FACFAS, Jon M. Humphers, DPM, and Daniel C. Jupiter, PhD
The association between hyperglycemia and postoperative complications has been well documented for many types of surgical procedures.1-11 Long-term glucose control, as measured by glycated hemoglobin, has been recognized as a risk factor for adverse outcomes following major surgeries, such as vascular and coronary artery procedures.1,2,5,10 In foot and ankle surgeries, poorly controlled diabetes and complicated diabetes have also been shown to be significant risk factors for both postoperative soft tissue (Figure 1) and bone healing (Figure 2) complications.12-16
Postoperative soft tissue complications
Poorly controlled diabetes has been shown to be a significant risk factor for postoperative infection following foot and ankle surgery.8-10 Myers and colleagues showed an association between an elevated glycated hemoglobin level and postoperative infections after hindfoot and ankle arthrodesis.12 For the purpose of their study, the cohort was dichotomized into patients with glycated hemoglobin of 7% or greater (indicating poorly controlled diabetes) and those with glycated hemoglobin of less than 7%. This case-control study found that patients with poorer glycemic control had a significantly higher postoperative infection rate than those with better control. (Table 1)
Younger et al also found that the most significant factor associated with successful transmetatarsal amputation (TMA) in diabetic patients was blood glucose control as measured by glycated hemoglobin.16 In their retrospective study of 42 patients, they compared mean glycated hemoglobin levels in patients with failed TMAs (defined as TMAs requiring surgical revision) with successful cases.
Among the many factors evaluated, the glycated hemoglobin level was the most significant. The mean level in the failed group was 10.6%, while that of the successful group was 7.8%. The authors emphasized the importance of obtaining glycated hemoglobin levels on initial consultation, and recommended postponing surgery until the level was below 8%, if possible, based on their bivariate analysis.
 Lepore et al evaluated patients admitted to the hospital for foot ulceration.17 In their cohort study, they compared glycated hemoglobin levels in patients who had major amputation (defined as amputation above the ankle), minor amputation, and no amputation. They found that patients who underwent amputation had significantly higher glycated hemoglobin levels than those who did not. In particular, those who underwent major amputation had a mean glycated hemoglobin level of 10%, while those with minor amputation or no amputation had mean glycated hemoglobin levels of 9% and 8%, respectively. In the same study, they also found that patients with lower glycated hemoglobin levels had lower ulcer grades at admission and fewer follow-up visits after discharge.
Lepore et al evaluated patients admitted to the hospital for foot ulceration.17 In their cohort study, they compared glycated hemoglobin levels in patients who had major amputation (defined as amputation above the ankle), minor amputation, and no amputation. They found that patients who underwent amputation had significantly higher glycated hemoglobin levels than those who did not. In particular, those who underwent major amputation had a mean glycated hemoglobin level of 10%, while those with minor amputation or no amputation had mean glycated hemoglobin levels of 9% and 8%, respectively. In the same study, they also found that patients with lower glycated hemoglobin levels had lower ulcer grades at admission and fewer follow-up visits after discharge.
Conversely, Aragón-Sánchez et al determined in their study on surgical treatment of osteomyelitis in diabetic feet that perioperative capillary blood glucose was a predictive factor for amputation, while long-term glycemic control was not found to be a significant factor.18 In this cohort study, they grouped the glycated hemoglobin level into first versus second to fourth quartiles. The first quartile of patients had glycated hemoglobin levels from 5.3% to 7.3% while those in the second to fourth quartiles had glycated hemoglobin levels ranging from 7.4% to 14%.
Postoperative bone healing complications
 Bone healing complications in the setting of diabetes have been extensively studied in animal models.19-43 In humans, incidence of bone healing complications in foot and ankle surgeries is known to be higher in people with diabetes than in those without diabetes.13,44-50 Although the association of hyperglycemia with bone healing complication has been well-documented,13,20,26,27,34,35,37,41,51,52 little clinical information is available as to which diabetes-related comorbidities directly affect the bone healing at a biochemical level. Studies of diabetic animal models, such as those mentioned above, have produced many theories as to what causes disturbance in normal bone metabolism, yet translational research is lacking.
Bone healing complications in the setting of diabetes have been extensively studied in animal models.19-43 In humans, incidence of bone healing complications in foot and ankle surgeries is known to be higher in people with diabetes than in those without diabetes.13,44-50 Although the association of hyperglycemia with bone healing complication has been well-documented,13,20,26,27,34,35,37,41,51,52 little clinical information is available as to which diabetes-related comorbidities directly affect the bone healing at a biochemical level. Studies of diabetic animal models, such as those mentioned above, have produced many theories as to what causes disturbance in normal bone metabolism, yet translational research is lacking.
In our own case-control study of diabetic patients, approximately one out of four patients had one or more bone healing complications.53 Bone healing complication was defined as the presence of one or more of the following: nonunion, malunion, delayed union, or surgical- or trauma-induced Charcot neuroarthropathy. We found patients with glycated hemoglobin levels of more than 7% had roughly three times greater odds of having a bone healing complication than those with glycated hemoglobin levels less than 7%. However, the most significant factor associated with bone healing complication in this diabetic cohort was the presence of neuropathy.
The diabetic patients with neuropathy had four times the odds of having a bone healing complication than diabetic patients without neuropathy, independent of glycated hemoglobin level. This result is consistent with many animal studies, as well as clinical reports on diabetic bone healing complications, which indicate that bone healing complications can be due to malfunctions of bone metabolism resulting from neuropathy.47,54-57
Interestingly, the osteolytic activities in Charcot processes in diabetic patients are also believed to be strongly linked to neuropathy (Figure 3) induced by diabetes, rather than to hyperglycemia itself. In fact, many Charcot cases have occurred in nondiabetic neuropathic populations.55,58-66 Lack of adequate neuropeptide release in a neuropathic patient may upregulate osteoclastogenesis while downregulating osteoblastic activity.54,67-69
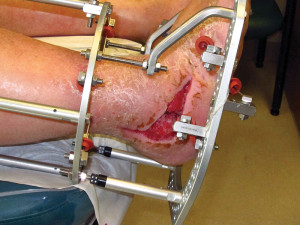
Figure 1. This man, aged 51 years, underwent Charcot reconstruction of the hindfoot and ankle. He developed a postoperative wound dehiscence on the lateral aspect of the foot, which required more than three months of local wound care for closure.
Although it is unclear whether neuropathy is the main cause of poor diabetic bone healing in foot and ankle surgeries, we have found that it has the strongest association with poor healing among all variables tested. Regardless of causality, since neuropathy is the major risk factor for diabetic bone healing complication, it becomes clear that long-term control of glucose is essential for preservation of normal nerve function.
Glycated hemoglobin levels
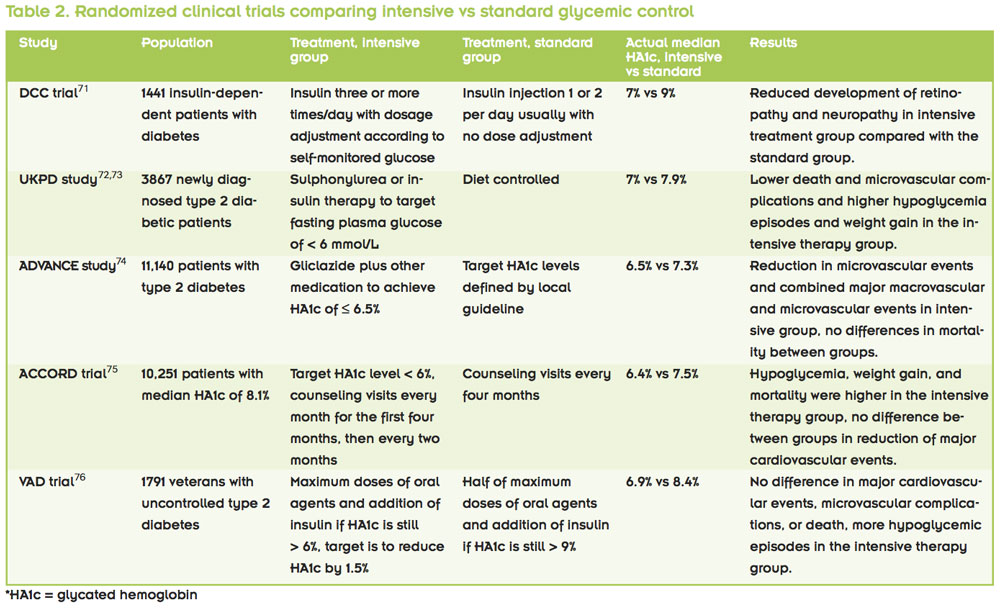
Most often in studies assessing the impact of long-term glycemic control on postoperative outcomes, glycated hemoglobin level is used as the metric for control. When comparing people with well-controlled versus poorly controlled diabetes, many researchers use a cutoff level of 7%, based on the American Diabetes Association (ADA) recommendation.70 The ADA recommendation is derived from several studies assessing intensive glycemic control therapy for reduction of long-term complications associated with diabetes (Table 2).
The Diabetes Control and Complications Trial Research Group (DCCT) study compared intensive therapy with conventional therapy in patients diagnosed with type 1 diabetes.71 The intensive therapy consisted of insulin administration three or more times daily, with dosage adjustment depending on the patient’s self-monitored glucose level. In contrast, the conventional group had two daily insulin injections, usually without dosage adjustment. The intensive therapy group had a median glycated hemoglobin level of around 7%, while that of the conventional group was around 9%. The investigators demonstrated that intensive glycemic control was associated with significant reduction in microvascular and neuropathic complications.
The UK Prospective Diabetes Study (UKPDS), showed similar effects of strict glycemic control in a cohort of type 2 diabetes patients in terms of reduction of microvascular and neuropathic complications.72,73 Their intensive therapy group had a median glycated hemoglobin level of 7%, while that of the conventional group was 7.9%.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study also compared intensive versus standard glycemic control groups in type 2 diabetes.74 In this study, the intensive treatment group had a target glycated hemoglobin level of less than 6.5%, while the standard group had their target level based on local guidelines. The investigators found a lower incidence of major microvascular events (mainly neuropathy) and combined major macro- and microvascular events in the intensive control group. There was no difference in mortality between the groups.
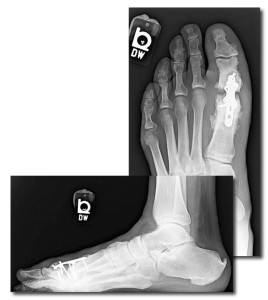
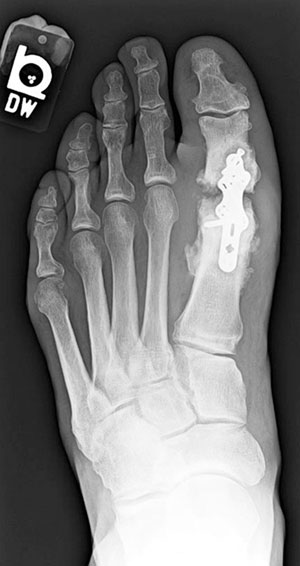
Figure 2. This man, aged 56 years, who had sensory neuropathy and received a new diagnosis of diabetes after surgery, underwent first metatarsophalangeal fusion with enhanced plate fixation. He developed nonunion of the joint mostly due to diabetic osteolysis, as evidenced by resorption at the joint. His postoperative glycated hemoglobin level (when diabetes was diagnosed) was 10.8%
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial compared a group with intensive intervention and a target glycated hemoglobin level of less than 6% against a standard therapy group with a target level of 7% to 7.9%.75 The actual median glycated hemoglobin levels achieved in these groups were 6.4% and 7.5%, respectively. In this study, the intensive intervention group actually had higher levels of mortality, weight gain, and hypoglycemia requiring assistance, while not showing any benefit in terms of cardiovascular risk reduction.
The Veterans Affairs Diabetes Trial (VADT) also compared an intensive intervention group with a standard therapy group in poorly controlled diabetic veterans.76 For this trial, researchers started the intensive therapy group on maximum doses of oral medication and initiated insulin when the glycated hemoglobin level was not reduced to less than 6% rather than considering a change in oral medication.
The goal for the intensive therapy was a 1.5% reduction in glycated hemoglobin levels. The standard therapy group started with half the maximal oral medication dose and did not begin insulin unless the level was above 9%. The actual median glycated hemoglobin levels were 6.9% and 8.4% in the intensive and standard therapy groups, respectively. The study found that there was no difference in risk of cardiovascular events, microvascular complications, or death between the groups. Hypoglycemic events were observed in 24% versus 18% of the patients in the intensive and standard treatment groups, respectively.

Figure 3. This woman, aged 52 years, with diabetes and sensory neuropathy with loss of protective sensation up to the level of the midleg, developed Charcot neuroarthropathy at the Lisfranc joint. This was subsequently treated surgically, but surgery was followed by another episode of Charcot changes in the same area and further collapse of the tarsometatarsal joints.
Summarizing these findings, the benefits of lowering glycated hemoglobin in diabetics in terms of reduction of diabetes-related macro- and microvascular complications appear to be substantial. However, in lowering glycated hemoglobin below 7%, the benefits seem to diminish, and there is also a risk of adverse events (including death, weight gain, and hypoglycemic episodes) in this range. It is perhaps for these reasons that the ADA chose 7% as its recommended cutoff.
Discussion
There is overwhelming evidence that a high glycated hemoglobin level is associated with poor outcomes in foot and ankle surgery. However, what constitutes an “acceptable” or “ideal” glycated hemoglobin level for foot and ankle surgery is still under investigation.
An acceptable glycated hemoglobin range for foot and ankle surgery can be interpreted to mean a range in which the risk of complication is no worse than for the nondiabetic population. On the other hand, one may be more interested in discovering a level at which the rate of complication spikes significantly; for example, if glycated hemoglobin levels of 6%, 7%, 8%, and 9% result in complication rates of 5%, 6%, 10%, and 11%, respectively, one may argue that the “spike” is found between 7% and 8%.
Alternately, the acceptable range can be defined as the level above which the risks associated with surgery become greater than those associated with nonsurgical treatment. In this case, the acceptable level for glycated hemoglobin may vary depending on the procedure. For example, incision and drainage of gas gangrene may have a higher acceptable glycated hemoglobin level than lateral ankle stabilization, simply because delaying surgical treatment of gas gangrene would have a more significant impact on the patient than delaying the ankle stabilization. Therefore, procedure-specific studies will be necessary to find the reference points.
In general, in elective cases, we would like to identify the level of glycated hemoglobin at which significantly more postoperative complications occur than in the population at large. For example, if the overall infection rate for a given elective foot and ankle procedure in the general population is 3%, we would like to know the level of glycated hemoglobin at which complication occurs more than 3% of the time. Future foot and ankle specific studies should focus on defining the “acceptable outcome” and identifying the associated glycated hemoglobin cutoff rather than simply comparing the levels of glycemic control between successful and failed groups.
Unfortunately, most studies in foot and ankle surgery to date are comparisons of glycated hemoglobin levels between groups with and without complications. Though they may tell us that people who experience complications have poorer glycemic control, they do not give us a useful reference point for glycemic control that clinicians can use for surgical decision-making.
On the other hand, some studies do evaluate the degree of association between perioperative glycemic control and postoperative complications. This information can be useful in assessing the chances of potential complication in diabetic patients when glycated hemoglobin levels are available to the surgeons. Still, this does not provide the ideal glycemic control level at which foot and ankle surgeries can be performed safely.
As recommended by many, including the ADA, a glycated hemoglobin level of 7% is a relatively good reference point, at least in terms of general health. However, foot and ankle surgery-specific evidence is lacking. Further, the cutoff line can also depend on the type of procedure to be performed. In addition, the acceptable range in emergent cases could be different than for minor elective cases.
We recommend obtaining a glycated hemoglobin measurement on initial consultation regardless of serum glucose, as it provides a good impression of potential postoperative sequelae. This is in contrast to a random glucose measurement, which can be influenced by a variety of preoperative stresses. Our approach to elective surgery in patients with uncontrolled diabetes mellitus is to delay surgery until better glycemic control can be obtained.
Although a glycated hemoglobin level of less than 7% may be ideal, it may not always be practical. For those patients with uncontrolled diabetes who require more urgent surgery, we recommend earlier and more frequent postoperative follow-up and increased vigilance for postoperative infections and early wound complications. A more thorough counseling session must be undertaken preoperatively to discuss other factors that may be predictive of postoperative complications. It should also be noted that intensive glycemic control itself could be a risk, especially in a chronically uncontrolled diabetic patient population. Caution should be taken when attempting aggressive preoperative hyperglycemic control.
Naohiro Shibuya, DPM, MS, FACFAS, is an associate professor of surgery at Texas A&M Health Science Center, College of Medicine; acting chief, Section of Podiatry, Department of Surgery, for the Central Texas VA Health Care System; and a staff podiatrist for the Scott and White Health Care System, all in Temple, TX. Jon M. Humphers, DPM, is chief resident in the Department of Podiatric Medicine and Surgery at Texas A&M Health Science Center, the Central Texas VA Health Care System, and the Scott and White Health Care System. Daniel C. Jupiter, PhD, is an assistant professor of surgery at Texas A&M Health Science Center, College of Medicine, and a research scientist in the Department of Surgery for the Scott and White Health Care System and the Central Texas VA Health Care System.
1. Acott AA, Theus SA, Kim LT. Long-term glucose control and risk of perioperative complications. Am J Surg 2009;198(5):596-599.
2. Dronge AS, Perkal MF, Kancir S, et al. Long-term glycemic control and postoperative infectious complications. Arch Surg 2006;141(4):375-380.
3. Glassman SD, Alegre G, Carreon L, et al. Perioperative complications of lumbar instrumentation and fusion in patients with diabetes mellitus. Spine J 2003;3(6):496-501.
4. Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999;22(9):1408-1414.
5. Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008;136(3):631-640.
6. Lamloum SM, Mobasher LA, Karar AH, et al. Relationship between postoperative infectious complications and glycemic control for diabetic patients in an orthopedic hospital in Kuwait. Med Princ Pract 2009;18(6):447-452.
7. Marchant MH Jr, Viens NA, Cook C, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am 2009;91(7):1621-1629.
8. Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol 2011;5(2):412-418.
9. Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007;156(1):137-142.
10. O’Sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1c) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 2006;32(2):188-197.
11. Richards JE, Kauffmann RM, Zuckerman SL, et al. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J Bone Joint Surg Am 2012;94(13):1181-1186.
12. Myers TG, Lowery NJ, Frykberg RG, Wukich DK. Ankle and hindfoot fusions: comparison of outcomes in patients with and without diabetes. Foot Ankle Int 2012;33(1):20-28.
13. Perlman MH, Thordarson DB. Ankle fusion in a high risk population: an assessment of nonunion risk factors. Foot Ankle Int 1999;20(8):491-496.
14. Wukich DK, Belczyk RJ, Burns PR, Frykberg RG. Complications encountered with circular ring fixation in persons with diabetes mellitus. Foot Ankle Int 2008;29(10):994-1000.
15. Wukich DK, Shen JY, Ramirez CP, Irrgang JJ. Retrograde ankle arthrodesis using an intramedullary nail: a comparison of patients with and without diabetes mellitus. J Foot Ankle Surg 2011;50(3):299-306.
16. Younger AS, Awwad MA, Kalla TP, de Vries G. Risk factors for failure of transmetatarsal amputation in diabetic patients: a cohort study. Foot Ankle Int 2009;30(12):1177-1182.
17. Lepore G, Maglio ML, Cuni C, et al. Poor glucose control in the year before admission as a powerful predictor of amputation in hospitalized patients with diabetic foot ulceration. Diabetes Care 2006;29(8):1985.
18. Aragón-Sánchez J, Lázaro-Martínez JL. Impact of perioperative glycaemia and glycated haemoglobin on the outcomes of the surgical treatment of diabetic foot osteomyelitis. Diabetes Res Clin Pract 2011;94(3):e83-e85.
19. Alblowi J, Kayal RA, Siqueira M, et al. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol 2009;175(4):1574-1585.
20. Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res 2002;20(6):1210-1216.
21. Black CT, Hennessey PJ, Ford EG, Andrassy RJ. Protein glycosylation and collagen metabolism in normal and diabetic rats. J Surg Res 1989;47(3):200-202.
22. Coords M, Breitbart E, Paglia D, et al. The effects of low-intensity pulsed ultrasound upon diabetic fracture healing. J Orthop Res 2011;29(2):181-188.
23. Follak N, Kloting I, Merk H. Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats. Diabetes Metab Res Rev 2005;21(3):288-296.
24. Follak N, Klöting I, Wolf E, Merk H. Improving metabolic control reverses the histomorphometric and biomechanical abnormalities of an experimentally induced bone defect in spontaneously diabetic rats. Calcif Tissue Int 2004;74(6):551-560.
25. Follak N, Klöting I, Wolf E, Merk H. Histomorphometric evaluation of the influence of the diabetic metabolic state on bone defect healing depending on the defect size in spontaneously diabetic BB/OK rats. Bone 2004;35(1):144-152.
26. Follak N, Klöting L, Wolf E, Merk H. Delayed remodeling in the early period of fracture healing in spontaneously diabetic BB/OK rats depending on the diabetic metabolic state. Histol Histopathol 2004;19(2):473-486.
27. Gandhi A, Beam HA, O’Connor JP, et al. The effects of local insulin delivery on diabetic fracture healing. Bone 2005;37(4):482-490.
28. Gandhi A, Doumas C, O’Connor JP, et al. The effects of local platelet rich plasma delivery on diabetic fracture healing. Bone 2006;38(4):540-546.
29. Gandhi A, Liporace F, Azad V, et al. Diabetic fracture healing. Foot Ankle Clin 2006;11(4):805-824.
30. Gebauer GP, Lin SS, Beam HA, et al. Low-intensity pulsed ultrasound increases the fracture callus strength in diabetic BB Wistar rats but does not affect cellular proliferation. J Orthop Res 2002;20(3):587-592.
31. Goto S, Fujii H, Kono K, et al. Carvedilol ameliorates low-turnover bone disease in non-obese type 2 diabetes. Am J Nephrol 2011;34(3):281-290.
32. Hamada Y, Kitazawa S, Kitazawa R, et al. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone 2007;40(5):1408-1414.
33. Hamada Y, Kitazawa S, Kitazawa R, et al. The effects of the receptor for advanced glycation end products (RAGE) on bone metabolism under physiological and diabetic conditions. Endocrine. 2010;38(3):369-376.
34. Kayal RA, Alblowi J, McKenzie E, et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 2009;44(2):357-363.
35. Kayal RA, Tsatsas D, Bauer MA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 2007;22(4):560-568.
36. Lange J, Barz T, Ekkernkamp A, et al. Gene expression profile in bone of diabetes-prone BB/OK rats fed a high-fat diet. Genes Nutr 2013;8(1):99-104.
37. Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003;144(1):346-352.
38. Macey LR, Kana SM, Jingushi S, et al. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am 1989;71(5):722-733.
39. Mathey J, Horcajada-Molteni MN, Chanteranne B, et al. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcif Tissue Int 2002;70(4):305-311.
40. Ogasawara A, Nakajima A, Nakajima F, et al. Molecular basis for affected cartilage formation and bone union in fracture healing of the streptozotocin-induced diabetic rat. Bone 2008;43(5):832-839.
41. Santana RB, Xu L, Chase HB, et al. A role for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes 2003;52(6):1502-1510.
42. Topping RE, Bolander ME, Balian G. Type X collagen in fracture callus and the effects of experimental diabetes. Clin Orthop Relat Res 1994;(308):220-228.
43. Tyndall WA, Beam HA, Zarro C, et al. Decreased platelet derived growth factor expression during fracture healing in diabetic animals. Clin Orthop Relat Res 2003;(408):319-330.
44. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am 2001;32(1):113-133.
45. Blotter RH, Connolly E, Wasan A, Chapman MW. Acute complications in the operative treatment of isolated ankle fractures in patients with diabetes mellitus. Foot Ankle Int 1999;20(11):687-694.
46. Connolly JF, Csencsitz TA. Limb threatening neuropathic complications from ankle fractures in patients with diabetes. Clin Orthop Relat Res 1998;(348):212-219.
47. Costigan W, Thordarson DB, Debnath UK. Operative management of ankle fractures in patients with diabetes mellitus. Foot Ankle Int 2007;28(1):32-37.
48. Jones KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br 2005;87(4):489-495.
49. Kristiansen B. Ankle and foot fractures in diabetics provoking neuropathic joint changes. Acta Orthop Scand 1980;51(6):975-979.
50. Prisk VR, Wukich DK. Ankle fractures in diabetics. Foot Ankle Clin 2006;11(4):849-863.
51. Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone 2010;46(1):148-154.
52. Mehta SK, Breitbart EA, Berberian WS, et al. Bone and wound healing in the diabetic patient. Foot Ankle Clin 2010;15(3):411-437.
53. Shibuya N, Humphers JM, Fluhman BL, Jupiter DC. Factors associated with nonunion, delayed union, and malunion in foot and ankle surgery in diabetic patients. J Foot Ankle Surg 2013;52(2):207-211.
54. La Fontaine J, Harkless LB, Sylvia VL, et al. Levels of endothelial nitric oxide synthase and calcitonin gene-related peptide in the Charcot foot: a pilot study. J Foot Ankle Surg 2008;47(5):424-429.
55. Shibuya N, La Fontaine J, Frania SJ. Alcohol-induced neuroarthropathy in the foot: a case series and review of literature. J Foot Ankle Surg 2008;47(2):118-124.
56. Jeffcoate WJ. Theories concerning the pathogenesis of the acute charcot foot suggest future therapy. Curr Diab Rep 2005;5(6):430-435.
57. Sinacore DR, Hastings MK, Bohnert KL, et al. Inflammatory osteolysis in diabetic neuropathic (charcot) arthropathies of the foot. Phys Ther 2008;88(11):1399-1407.
58. Radhakrishnan K, Vijayan VP, Ashok PP, et al. Syphilitic spinal neuroarthropathy with paraplegia. Clin Neurol Neurosurg 1985;87(1):61-64.
59. Crim JR, Bassett LW, Gold RH, et al. Spinal neuroarthropathy after traumatic paraplegia. AJNR Am J Neuroradiol 1988;9(2):359-362.
60. Heylen Y. Neuropathic arthropathy of the shoulder secondary to syringomyelia. J Belge Radiol 1993;76(4):232-233.
61. Thornhill HL, Richter RW, Shelton ML, Johnson CA. Neuropathic arthropathy (Charcot forefeet) in alcoholics. Orthop Clin North Am 1973;4(1):7-20.
62. Sudanese A, Paderni S, Guerra E, Bertoni F. Neurogenic arthropathy of the knee due to chronic alcoholism: two case reports. Chir Organi Mov 2003;88(4):427-434.
63. Barber DB, Janus RB, Wade WH. Neuroarthropathy: an overuse injury of the shoulder in quadriplegia. J Spinal Cord Med 1996;19(1):9-11.
64. Horibe S, Tada K, Nagano J. Neuroarthropathy of the foot in leprosy. J Bone Joint Surg Br 1988;70(3):481-485.
65. Bjorkengren AG, Weisman M, Pathria MN, et al. Neuroarthropathy associated with chronic alcoholism. AJR Am J Roentgenol 1988;151(4):743-745.
66. Nagano J, Tada K, Masatomi T, Horibe S. Arthropathy of the wrist in leprosy—what changes are caused by long-standing peripheral nerve palsy? Arch Orthop Trauma Surg 1989;108(4):210-217.
67. Zaidi M. Neural surveillance of skeletal homeostasis. Cell Metab 2005;1(4):219-221.
68. Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech 2002;58(2):85-90.
69. Young MJ, Marshall A, Adams JE, et al. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 1995;18(1):34-38.
70. American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care 2013;36(Suppl 1):S11-S66.
71. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329(14):977-986.
72. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837-853.
73. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):854-865.
74. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358(24):2560-2572.
75. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358(24):2545-2559.
76. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360(2):129-39.