
iStockphoto.com 452546761
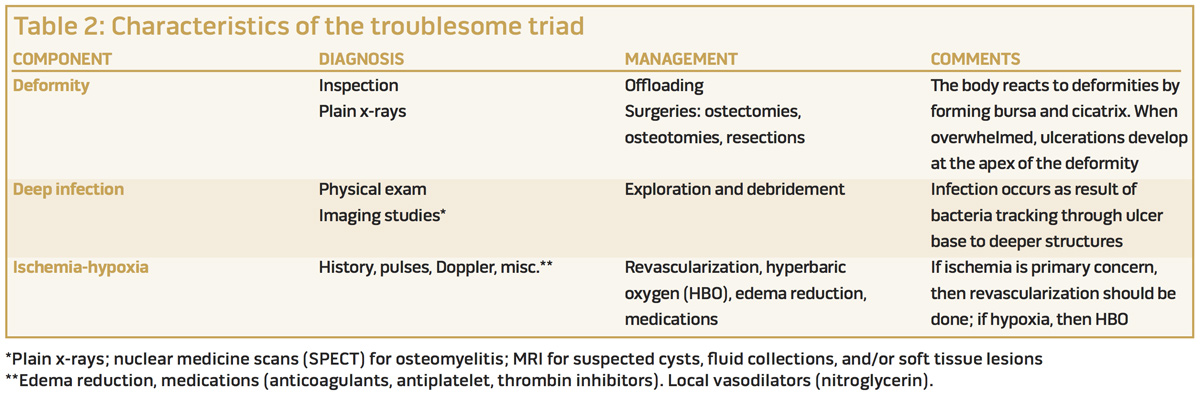
Uncontrolled deformity, deep infection, and ischemia-hypoxia make up the troublesome triad of confounders associated with healing challenges in patients with diabetic foot ulcers. Clinical examination and intervention in nonhealing patients should focus on these three elements.
By Anna Maria M. Tan, DPM; Michael B. Strauss, MD; and Lientra Q. Lu, BS
Diabetic foot ulcers (DFUs) are a common cause of morbidity and often require comprehensive multidisciplinary management. The cost of care imposes substantial economic burdens on the healthcare system. It adds an additional $9 billion to $13 billion dollars to the annual $245 billion spent for care of patients with diabetes mellitus in the US.1 DFUs also are a major risk factor for lower extremity amputations.2 The progression from preulcer to ulceration to infected nonhealing wound is a common course of events that in about 25% of cases leads to lower limb amputation.3
The majority of DFUs heal uneventfully with management that includes appropriate wound dressings, debridement, and offloading. There are usually identifiable reasons why a DFU does not heal. Often these reasons are multifactorial. We have observed that three confounding factors are responsible for failure of wound healing in more than 90% of cases.4 We term these factors—uncontrolled
deformity, deep infection, and ischemia-hypoxia—the “troublesome triad.” Being able to identify these elements of the troublesome triad is essential to the evaluation of diabetic wounds, their management, and determining outcomes.
We grade outcomes on all wounds on a 0-to-2 scale as follows:
1) Healed = 2 points
2) Improved (chronic stable wounds, with patients pain-free, able to resume activities, minimal dressing changes, healthy-appearing wound base) = 1.5 points
3) No change (not good enough for inpatients but may be acceptable for outpatients) = 1 point
4) Worsening = .5 point
5) Lower limb amputation or death = 0 points
We consider healed or improved outcomes to be positive, and no change, worsening, or lower extremity amputation or death to be poor outcomes.
A variety of wound-dressing agents are available to help with wound management of diabetic foot ulcers, such as negative pressure wound therapy (NWPT), subatmospheric wound dressings, bioengineered wound coverings, antimicrobials (including those with silver), medica-grade honey, agents that absorb secretions, or combinations of these. However, when elements of the troublesome triad are present, healing is unlikely even if the most advanced therapies are used. Advanced wound therapies such as biologic agents and NPWT should only be used after the confounders of the troublesome triad have each been addressed.
The elements of the troublesome triad are detailed in Figure 1 and described in more detail below.
Uncontrolled deformity
Foot deformities can cause plantar pressures to concentrate in a focal area and can create biomechanical stresses, both of which contribute to DFUs.5 Soft tissue breakdown follows repetitive cycles of pressure concentrations, shear stresses, or both, especially in patients with sensory neuropathy.6 Deformities we frequently observe in the diabetic foot include claw, hammer, and mallet toes; hallux valgus, varus, or rigidus; forefoot abduction/adduction; midfoot pronation/supination; plantarward extrusion of midfoot bones; equinus contracture; rocker bottom foot; and combinations of these conditions.
Bony prominences underlying mal perforans ulcers often are a consequence of the deformities and are a reason DFUs do not heal.7 In our experience, deformities can also occur secondary to Charcot neuroarthropathy bone destruction, motor components of peripheral neuropathy, malunion, cicatrix, and hypertrophic bursa formation. These complications often overshadow the extent of the underlying bony spur.
Cicatrix and bursa formation can develop as a type of defense mechanism by the body to provide padding over a deformity.7 In our experience, this is often self-defeating, with the amount of cicatrix and/or bursa far exceeding the magnitude of the bone deformity. When the mass effect of the cicatrix and hypertrophic bursa exceeds the protection the padding attempts to provide, an ulceration develops.
If the DFU is superficial, coexisting deformity may be only a mechanical problem and may not contribute to deep infection. In such cases, we recommend surgical management of the deformity when the ulcer persists after a trial of offloading and/or protective footwear, with as much surgical attention being focused on removing the cicatrix and bursa as on eliminating the underlying bony deformity.
Total contact casting (TCC) is recommended for outpatient management of diabetic foot ulcers.8 However, Frigg et al found an ulcer recurrence rate of 57% in patients treated with TCC, despite healing and compliant use of protective footwear.9 When the ulcers occur in the midfoot and hindfoot, and especially if hospital management of the deformity is required, TCC is usually not sufficient and surgery is required.9
Deep infection
The second component of the troublesome triad is infection, which frequently occurs with diabetic foot ulcers. In an Institutional Review Board-approved prospective study of patients hospitalized with DFUs, we found residual deep infection was the most frequent confounder, being present in 61.3% of the patients.4
Infections that are pertinent to this component of the troublesome triad are those that are deep (ie, involving bursa, cicatrix, and/or bone) to the skin and base of the wound and involve bone, bursa, cicatrix, or combinations of these. These deep infections typically do not respond to antibiotics and require surgical debridement to achieve healing. Failure to adequately debride the tissues affected by deep infection often, in our experience, leads to nonhealing and the need for lower limb amputation.7 Snyder reported in a retrospective study of diabetic patients with forefoot amputations that uncontrolled infection at the amputation site, even when concurrent or previously managed, was the reason 37% of the patients subsequently required a transtibial or above knee amputation.10
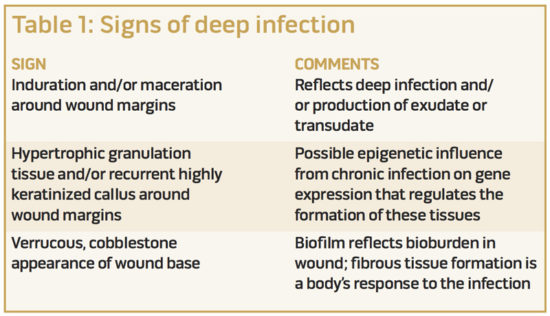
We have observed that deep infection includes persistence of infection even when treated with antibiotics and superficial debridement; induration and/or maceration around the wound margins; hypertrophic granulation tissue and/or recurrent highly keratinized callus around wound margins; persistent fibrous membranes and/or biofilm; a verrucous-cobblestone appearance or pale, atrophic appearance of the ulcer base; or combinations of these (Table 1). The hallmark of deep infection is the recurrence of findings at return clinic visits even when the wound appears improved after the superficial debridement. Often, these DFUs have been managed with a variety of advanced therapies before the decision is made to explore and debride the deep infection associated with bone, bursa, or cicatrix.
 Plain x-rays and nuclear medicine scans using a combination of indium and technetium and augmented with computed tomography are useful in identifying the source of a deep infection.11,12 Magnetic resonance imaging (MRI) is helpful in delineating the soft tissue components of the infection, but tends to be over-read, as the interpretation of bone infection is based on bone edema.13 Edema in bone can arise from inflammation of surrounding tissues and be interpreted as osteomyelitis on the MRI. Often, the radiologist will conclude “possible osteomyelitis” and suggest confirmation with a nuclear medicine study.
Plain x-rays and nuclear medicine scans using a combination of indium and technetium and augmented with computed tomography are useful in identifying the source of a deep infection.11,12 Magnetic resonance imaging (MRI) is helpful in delineating the soft tissue components of the infection, but tends to be over-read, as the interpretation of bone infection is based on bone edema.13 Edema in bone can arise from inflammation of surrounding tissues and be interpreted as osteomyelitis on the MRI. Often, the radiologist will conclude “possible osteomyelitis” and suggest confirmation with a nuclear medicine study.
The definitive diagnosis of osteomyelitis is made with culture and sensitivity of a bone sample.14 Probing to bone has an 85% sensitivity for osteomyelitis.15
Surgical management of deep infection requires removal of the infected bone, bursa, and cicatrix. Usually, all three components are involved when infection is the reason for nonhealing in a diabetic foot ulcer. Following adequate debridement, we recommend antibiotics be continued for a couple of weeks after surgery to sterilize the soft tissues adjacent to the debrided bone and soft tissues.
The use of hyperbaric oxygen (HBO) as an adjunct to management of deep infection associated with a DFU requires careful consideration. If ischemia-hypoxia is evident, it is possible the host factors will not be able to sterilize any residual infection in the bone or soft tissues, as the neutrophil-oxidative killing of bacteria and bone resorption by osteoclasts is highly oxygen-dependent. Hunt et al showed that 30-40 mm Hg oxygen tensions in the wound are required for healing to occur.16 At lesser oxygen tensions, the tissues may not die, but will be unable to heal the wound or eliminate the infection.17 HBO should be used as an adjunct to surgical and antibiotic management in such situations.
Ischemia-hypoxia
Perfusion that is not sufficient to meet oxygen requirements for wound healing and infection control is the third troublesome triad confounder. Pompers et al reported that 50% of patients with diabetic foot ulcers exhibit a component of ischemia.18 And Apelqvist et al found that the likelihood of wound healing without a major amputation is inversely related to the severity of underlying peripheral arterial disease (PAD), in addition to the seriousness of the patient’s comorbidities and the complexity of tissue involvement.19
The evaluation for PAD starts with a patient history and checking for symptoms of intermittent claudication and rest pain; these symptoms, however, may not be apparent because of sensory neuropathy. Components of the physical exam include checking for palpable pulses at the hip, knee, and ankle levels, as well as for secondary signs of perfusion such as pedal hair growth, skin quality, coloration, temperature, and toe capillary refill time. In the absence of palpable pulses, wound ischemia-hypoxia can be confirmed with Doppler imaging.20
Based on clinical signs and symptoms, imaging studies and possibly transcutaneous oxygen measurements (TCOMs) can be done to screen for the severity of PAD and provide justification for interventions. PAD is a prominent risk factor for lower extremity amputation regardless of etiology.21
When diabetic foot ulcers fail to improve in the context of the clinical findings noted above, angiography is the next step in the evaluation of critical limb ischemia-hypoxia. Comparing juxta-wound TCOMs under normal indoor air conditions and with hyperbaric oxygen can provide objective data with which to determine whether hyperbaric oxygen is needed for wound healing in these situations. If the TCOM readings exceed 40 mm Hg in room air, wound oxygenation is sufficient for wound healing, and failure to heal is likely due to one of the other confounders, or an occasional biochemical problem (for example, matrix metalloproteins). If the TCOMs are lower than 30 mm Hg, healing is not likely to occur. However, in cases where the juxta-wound TCOMs increase to more than 200 mm Hg with HBO exposure, regardless of the room air readings, we have observed healing in 88% of our patients,22 and similar findings were reported by Fife et al.23
Other interventions to mitigate wound ischemia-hypoxia should not be overlooked in this cohort of patients. These include edema reduction, optimization of cardiac function, and use of medications to improve blood rheology. Edema increases the diffusion distance of oxygen from the capillary to the cell along a gradient.24 Improving cardiac function increases perfusion to the ischemic tissues.
Finally, rheological agents such as aspirin, clopidogrel, warfarin, heparin, pentoxifylline, and dextran improve perfusion through anticoagulation, decreasing the sludging of erythrocytes in the microcirculation, and/or improving red blood cell deformity.25 When used as the only technique to improve perfusion, in our experience, they will not likely be adequate to achieve healing in an ischemic-hypoxic wound; consequently, we recommend they be used in conjunction with the other methods described.
Conclusions
Uncontrolled deformity, deep infection, and ischemia-hypoxia are the three elements that we have labeled the troublesome triad, and these confounders are associated with healing challenges in patients with DFUs (Table 2). Our prospective study found one or more troublesome triad confounders in 91.9% of 62 inpatients with DFUs.4
Each confounder has characteristic findings that can be confirmed with examination and remedied with interventions. These include surgical removal of the deformity and stabilization of the foot in a plantigrade position; debridement of infected bone, bursa, and cicatrix; and improving perfusion with revascularization techniques, hyperbaric oxygen, and medical interventions. Our 0-to-2 point grading system for wound outcomes is a useful tool to assess the effectiveness of our interventions to manage these cases.
We have observed “good” outcomes in nearly 80% of our patients (unpublished data) hospitalized with diabetic foot wounds when elements of the troublesome triad were addressed.
 We feel it is incumbent on clinicians treating these patients to recognize the components of the troublesome triad and to correct them before trying to achieve wound healing with other interventions, particularly those that are costly, as healing is unlikely to occur and persist when these confounders have not been addressed. In particular, interventions to target the troublesome triad confounders should not be ignored or deferred in favor of using advanced wound therapies. Recognition and management of these confounders can help conserve resources when attempting to heal diabetic foot ulcers.
We feel it is incumbent on clinicians treating these patients to recognize the components of the troublesome triad and to correct them before trying to achieve wound healing with other interventions, particularly those that are costly, as healing is unlikely to occur and persist when these confounders have not been addressed. In particular, interventions to target the troublesome triad confounders should not be ignored or deferred in favor of using advanced wound therapies. Recognition and management of these confounders can help conserve resources when attempting to heal diabetic foot ulcers.
In addition, it is important to recognize that not all wounds heal, and in our clinical experience, a patient may live with a chronic stable wound for several years. Addressing the troublesome triad allows clinicians to manage these chronic stable wounds with glycemic control monitoring and medication.
Anna Maria M. Tan, DPM, is chief resident of Podiatric Medicine & Surgery at Long Beach Memorial Medical Center in California. Michael B. Strauss, MD, is the former director of Hyperbaric Medicine at Long Beach Memorial Medical Center. Lientra Q Lu, BS, is a research coordinator at VA Medical Center Long Beach Healthcare System and administrative assistant at the Southern California Institute for Research and Education in Long Beach.
References
- Boyko EJ, Ahroni JH, Stensel V, et al. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999;22(7):1036-1042.
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: Basis for prevention. Diabetes Care 1990;13(5):513-521.
- Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26(2):491-494.
- Strauss MB, Moon H, La SS, et al. The incidence of confounding factors in patients with diabetes mellitus hospitalized for diabetic foot ulcers. Wounds 2016;28(8):287-294.
- Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 1992;35(7):660-663.
- Thompson DE. The effects of mechanical stress on soft tissue. In: Levin ME, O’Neal LW, eds. The Diabetic Foot. 4th ed. Maryland Heights, MO; Mosby Publishing Company: 1988.
- Strauss MB, Manji KM, Miller SS, Manji A. Bursa and callus: friend or foe. Wound Care Hyperbaric Med 2013;4(2):19-28.
- Coughlin MJ, Mann RA, Saltzman CL. Surgery of the foot and ankle. Volume II. 8th ed. Philadelphia, PA; Mosby Elsevier: 2007; 1303-1304.
- Frigg A, Pagenstert G, Schafer D, et al. Recurrence and prevention of diabetic foot ulcers and total contact casting. Foot Ankle Int 2007;28(1):64-69.
- Snyder DC, Salameh JR, Clericuzio CP. Retrospective review of forefoot amputations at a Veterans Affairs hospital and evaluation of post-amputation follow-up. Am J Surg 2006;192(5):e51-e54.
- Johnson JE, Klein SE, Brodsky JW. Diabetes. In: Coughlin MJ, Saltzman CL, Anderson RB, eds. Mann’s surgery of the foot and ankle. 9th ed. Amsterdam, Netherlands; Elsevier Saunders: 2014; 1385-1480.
- La Fontaine J, Bhavan K, Lam K, et al. Comparison between Tc-99m WBC SPECT/CT and MRI for diagnosis of biopsy-proven diabetic foot osteomyelitis. Wounds 2016;28(8):271-278.
- Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg 2009;23(2):80-89.
- Elamurugan TP, Jagdish S, Kate V, Chandra Parija S. Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis. Int J Surg 2011;9(3):214-216.
- Grayson ML, Gibbons GW, Balogh K, et al. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273(9):721-723.
- Hunt TK, Zederfeldt B, Goldstick TK. Oxygen and healing. Am J Surg 1969;118(4):521-525.
- Hohn DC and Hunt TK. Oxidative metabolism and microbicidal activity of rabbit phagocytes: cells from wounds and peripheral blood. Surgical Forum 1975;26:85-87.
- Pompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50(1):18-25.
- Apelqvist J, Elgzyri T, Larsson J, et al. Factors related to outcome of neuroischemic/ischemic foot ulcer in diabetic patients. J Vasc Surg 2011;53(6):1582-1588.
- Wagner, W. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981;2(2):64-122.
- Nerone VS, Springer KD, Woodruff DM, Atway S. Reamputation after minor foot amputation in diabetic patients: risk factors leading to limb loss. J Foot Ankle Surg 2013;52(2):184-187.
- Strauss MB, Bryant BJ, Hart GB. Transcutaneous oxygen measurements under hyperbaric oxygen conditions as a predictor for healing of problem wounds. Foot Ankle Int2002; 23(10):933-937.
- Fife CE, Buyukcakir C, Otto GH, et al. The predictive value of transcutaneous oxygen tension measurement in diabetic lower extremity ulcers treated with hyperbaric oxygen therapy: a retrospective analysis of 1144 patients. Wound Repair Regen 2002;10(4):198-207.
- Strauss MB, Aksenov IV, Miller SS, et al. Mechanisms of hyperbaric oxygen: Secondary tissue consequences of hyperoxygenation and pressurization. Wound Care Hyperbaric Med 2012;3(4):45-63.
- Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987;34(1):50-97.










