Current treatments for chronic ankle instability (CAI) may be ineffective in reducing its development and recurrence. To help address this issue, the authors have proposed a new treatment paradigm based on a theoretical model of CAI as an organismic constraint on the sensorimotor system.
By Patrick O. McKeon, PhD, ATC, CSCS, and Erik A. Wikstrom, PhD, ATC, FACSM
Ankle sprains are the most common injuries associated with physical activity and athletic participation,1,2 accounting for approximately 60% of all injuries that occur during interscholastic and intercollegiate sports.1-5 Medical costs for ankle sprains have been estimated to be approximately $4 billion annually.6,7 Thus ankle sprains, while often viewed as mild injuries, represent a significant public health problem7,8 and a major healthcare burden.
Further, about 30% of those who suffer a first time ankle sprain develop chronic ankle instability (CAI); some have reported this number to be as high as 75%.9-11 This means at least one out of every three individuals who sprain an ankle will go on to suffer residual symptoms indefinitely. Indeed, the residual symptoms that define CAI significantly alter an individual’s health and function by causing them to become less active over time. CAI is also a major contributing factor in the development of post-traumatic ankle osteoarthritis,12 for which there are no effective conservative treatments.
It is apparent, based on the information presented above, that CAI presents a major obstacle affecting the maintenance of regular physical activity for Americans and the prevention of post-traumatic ankle osteoarthritis. Further, high recurrence rates,9-11 incidence of post-traumatic ankle osteoarthritis,12 and consequent healthcare burdens6,7 clearly indicate that current treatments for CAI may be ineffective in reducing its development and recurrence. To help address this issue, we have proposed a new treatment paradigm based on a theoretical model of CAI as an organismic constraint on the sensorimotor system.
The theoretical model

Figure 1. The interacting constraints that influence sensorimotor control. Sensorimotor system (SMS) freedom is contextually dependent on the interaction of the organism, the complexity of the task, and the predictability of the environment.
The human body is a system capable of accomplishing movement goals in a variety of ways.13 The dynamic nature of this system (ie, its ability to adapt to changing demands) is often described by what is referred to as the dynamic systems theory of motor control. According to this theory, the organization of the sensorimotor system is constrained, or shaped, by the interaction of organismic, task, and environmental constraints (see Figure 1).13,14 The theory states that the sensorimotor system develops and changes strategies (ie, self-organizes) based on its current state and on interactions with the environment as they relate to a particular movement goal.14 Thus, a healthy sensorimotor system can accomplish a movement goal in a variety of ways even if changes in the task, environment, or both occur. Increasing numbers of possible solutions (ie, degrees of freedom) translate to an enhanced ability to successfully accomplish the movement goal and cope with change.
CAI can be viewed as an organismic constraint, potentially because the damaged mechanoreceptors in the lateral ligaments cannot be used for movement solution development, thus reducing the functional variability and ability of the sensorimotor system to accomplish movement goals.15 This constraint has been proposed to increase the risk of recurrent injury.14 Injury epidemiological evidence supports this framework in that the primary risk factor for an ankle sprain is a previous history of the same injury.16 Further, poor sensorimotor control is also a risk factor for ankle injury.17
The hallmark of CAI is self-reported disability in combination with experiences of the ankle giving way during functional activities.18 Those with CAI have also demonstrated alterations in both sensory and motor aspects of sensorimotor control, including plantar sensation threshold deficits,21 increased joint position sense errors,22 decreased dorsiflexion range of motion,21, 22 impaired muscle reaction time,23 decreased balance,24 and gait and landing alterations.25-29 Thus, it appears that there is a continuum of disability (see Figure 2) associated with sensorimotor control that modulates self-reported activity limitations and participation restrictions experienced by those with CAI.15,30

Figure 2. The continuum of disability. Ankle sprains introduce increased organismic constraints to the body, which in turn impair sensorimotor control. This in turn decreases functional performance, which increases the risk of a subsequent ankle sprain.
To gain understanding into this continuum, research is needed to elucidate the link among constraints, sensorimotor control, and injury risk.
While the exact neurophysiologic mechanism of CAI remains unknown, it has been hypothesized that damaged structures from the ankle sprain relay absent or inappropriate afferent information to the central nervous system (ie, sensory dysfunction).31 In healthy individuals, organization of movement solutions arises from the abundance of degrees of freedom from both sensory and motor sources.13 As a movement is executed, sensory information “tunes” motor output, and actual movement itself serves to “tune” sensory input. If a source of sensory input is impaired, as in the condition of CAI, the sensorimotor system dynamically reprioritizes other inputs available to compensate for the loss of information,32 as we have previously shown.33,34 We have evidence to support this hypothesis;21,27, 35-41 that this dysfunction limits an individual’s ability to self-organize (ie, cope with changes in the environment or with increased activity demands) due to an inability to appropriately modulate movement based on information from internal and external sources (see Figure 3). The consequence is a reduction in the total degrees of freedom available from sensory inputs to detect and cope with change.
It may be that the motor issues related to CAI—including poor balance, gait alterations, and episodes of giving way—are related to sensory deficits. This is consistent with the original hypothesis of Freeman et al,31 who suggested the recurrent instability that those with CAI experience was due to deafferentation of the lateral ankle ligaments as a consequence of injury. This loss of afferent information was then thought to manifest as the diminished ability to balance on one leg and as self-reported episodes of instability. Therefore, the constraints experienced by those with CAI may be related to alterations in the sensory degrees of freedom available to the sensorimotor system for tuning motor output.
The paradigm shift

Figure 3. The relationship of sensory and motor impairments associated with CAI. On the sensory side, deficits in plantar information, joint position sense, and range of motion may contribute to alterations associated with the motor output of the system. Typically, research has evaluated the quality of motor output as it relates to CAI; however, a growing body of literature suggests sensory issues are present and may be driving the dysfunction associated with the condition.
Traditionally, rehabilitation strategies for CAI have focused solely on motor impairments within the sensorimotor system, with little attention paid to the role of sensory inputs in the regulation of function. We hypothesize that, due to the CAI-related disruption of sensory information, the sensorimotor system is more reliant on other sources of somatosensory input (ie, plantar cutaneous receptors, muscle spindles, posterior ankle articular receptors) available to tune movement.33 From a dynamic systems perspective, targeting and manipulating sensory information sources will have a very beneficial effect on rehabilitation outcomes in those with CAI.42 Targeting sources of sensory information will allow the patient to optimize movement patterns to cope with changing conditions—a marked limitation of existing treatment paradigms that target only motor impairments. Investigating the effect of therapeutic interventions that address this sensory phenomenon may offer individuals with CAI the opportunity to better self-organize (ie, compensate) for the loss of sensory information from damaged structures and resolve motor impairments.
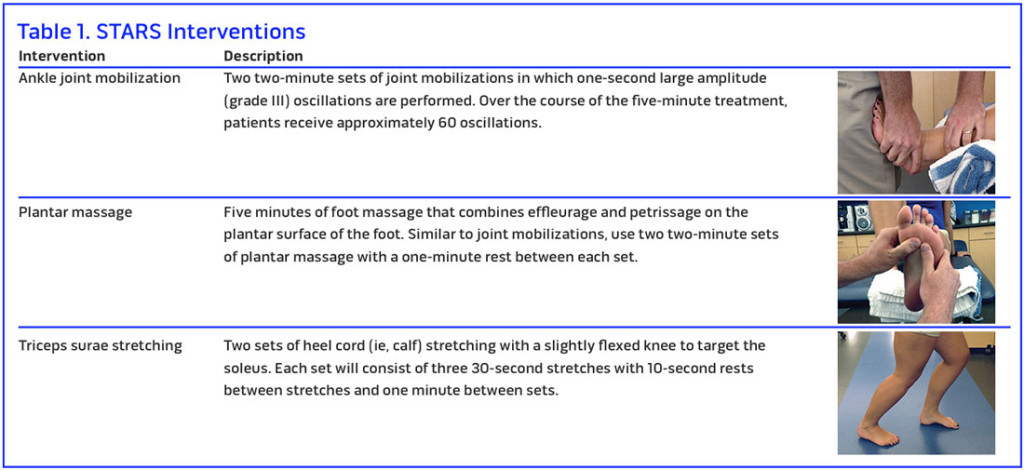
Manual therapies, such as ankle joint mobilizations, plantar massage, and stretching, are hypothesized to target specific sensory inputs such that CAI-associated dysfunction will improve through enhanced afferent and global function (see Figure 4). Targeting certain sensory pathways to improve the quality and quantity of sensory information provided to the sensorimotor system may enhance the output on the motor side of the system. This has led us to the development of a new treatment paradigm for CAI known as sensory-targeted ankle rehabilitation strategies (STARS). In this paradigm, we have explored the effects of ankle joint mobilizations, plantar massage, and triceps surae stretching on patient-, clinician-, and laboratory-based outcome measures that are representative of sensorimotor function and disability in those with CAI. The outcome of this research has potential to generate a paradigm shift for treatment strategies in this population.
STARS interventions and initial evidence

Figure 4. The STARS project focuses on examining how manual therapies at each sensory entry point affect the motor output and functional performance of those with CAI. By developing an understanding about how these treatments affect the system, more robust treatment strategies for CAI patients can be tailored to their sensory issues.
The STARS interventions include ankle joint mobilization, plantar massage, and triceps surae strengthening. (See Table 1 for images and additional descriptions of each modality.)
Ankle joint mobilization. In the STARS paradigm, we and others have begun using anterior-to-posterior Maitland grade III joint mobilizations. As an example, we are using a treatment period of five minutes to stimulate the surrounding ankle capsuloligamentous receptors. Each treatment consists of two sets of joint mobilizations, two minutes each, in which one-second grade III oscillations are performed, with one minute of rest between sets. While the parameters might vary with regard to number of oscillations, patient positioning, and number of treatments, ankle joint mobilizations consistently improve ankle joint range of motion,43,44 as well as postural control and self-reported function,43 in patients with CAI. A recent systematic review has also demonstrated improvements in patient-, clinician-, and laboratory-based outcomes with greater effectiveness with multiple treatments compared with single treatments in those with a history of ankle sprains.45
Plantar massage. The plantar cutaneous receptors play an important role in the maintenance of postural control and those with CAI may rely more heavily on their information. To capitalize on this source of information, we built a protocol in which patients receive five minutes of foot massage, which combines effleurage and petrissage techniques, on the plantar surface of the foot. Similar to joint mobilizations, we have used two two-minute sets of plantar massage with one minute of rest in between each set. Previously, a similar protocol demonstrated that those with CAI experienced improved postural control after undergoing a single treatment session.46 Similar results have also been shown in the elderly, a patient population that also has organismic constraints.47
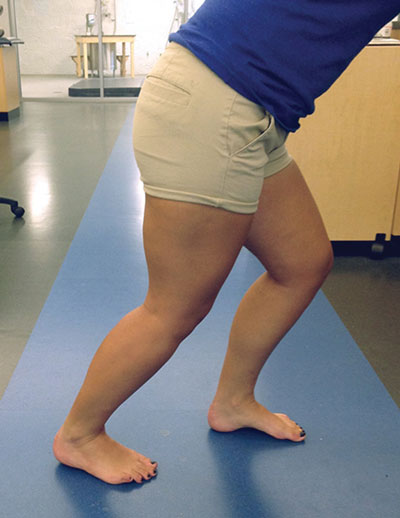
Triceps surae stretching. Research also suggests the muscle spindles within the gastrocnemius-soleus complex (triceps surae) are important for maintenance of postural control.48,49 Using the STARS paradigm, it would be possible to use this treatment modality to improve outcomes associated with a history of ankle sprains. In our study, we used a protocol of two sets of heel cord (calf) stretching. Each set consisted of three 30-second stretches, with 10 seconds of rest between stretches and one minute between sets. While little research has been done on the effectiveness of stretching in those with CAI, a recent systematic review demonstrated that stretching was an effective modality for improving dorsiflexion range of motion in those with a history of ankle sprains.50
To date, a number of studies from several different labs have demonstrated the effectiveness of different sensory-targeted ankle rehabilitation strategies on a variety of patient-, clinician-, and laboratory-based outcomes.51 However, no investigation has systematically compared different STARS interventions against each other on a wide range of outcome measures. Thus, the purpose of our investigation was to determine the effectiveness of STARS for facilitating immediate and prolonged improvements in subjective and objective outcome measures of clinical disablement and sensorimotor dysfunction in those with CAI. More information about this study (NIAMS 5R03AR061561) can be found at clinicaltrials.gov. Specific outcome measures for the STARS project included the changes due to each STARS on the Foot and Ankle Ability Measure52 and self-reported episodes of giving way, the weight-bearing lunge test,43 the single-limb balance test,53 time-to-boundary measures of single limb balance,34,36 gait initiation,54,55 and plantar cutaneous sensitivity.56
Future research and clinical implications
There are several important clinical questions that will need to be answered regardless of the existing literature and our own results. As a result, future research is needed to determine the dosage needed to optimally improve subjective and objective sensorimotor outcomes in patients with CAI. Similarly, while STARS treatment has been associated with improvement in a number of outcomes, there remain an even larger number of possible variables that may better illustrate how STARS results in improved function for those with CAI. Most important, in the age of personalized medicine, future research is needed to establish predictive criteria that would allow a clinician to select the most effective intervention or interventions for those with CAI based on a simple initial evaluation. Lastly, the existing knowledge and our results will begin to form a framework for the systematic investigation of combination therapies for patients with CAI, such as combining STARS with more traditional motor-focused interventions such as balance training.
Clinical take-home points:
- The sensorimotor system relies on both sensory and motor sources to tune its behavior in the presence of changing task and environmental demands.
- Damaged or limited sensory information may result in a more constrained sensorimotor system.
- By targeting certain sensory pathways through manual therapy interventions, it may be possible to provide the sensorimotor system greater freedom to cope with change and enhance functional performance in those with CAI.
- The STARS interventions are delivered across a five-minute treatment session six times over the course of two weeks. These low-cost treatment strategies can be implemented in any healthcare setting with little to no equipment needed.
Patrick O. McKeon, PhD, ATC, CSCS, is the clinical education coordinator within the Athletic Training Education Program at Ithaca College in New York. Erik A. Wikstrom, PhD, ATC, FACSM, is an associate professor within the Department of Kinesiology at the University of North Carolina at Charlotte.
Disclosure: This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, grant #5R03AR061561.
- Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med 2007;14(7):641-645.
- Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train 2007;42(2):311-319.
- Almeida SA, Williams KM, Shaffer RA, Brodine SK. Epidemiological patterns of musculoskeletal injuries and physical training. Med Sci Sports Exerc 1999;31(8):1176-1182.
- Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med 2007;37(1):73-94.
- Powell JW, Barber-Foss KD. Injury patterns in selected high school sports: A review of the 1995-1997 seasons. J Athl Train 1999;34(3):277-284.
- Curtis CK, Laudner KG, McLoda TA, McCaw ST. The role of shoe design in ankle sprain rates among collegiate basketball players. J Athl Train 2008;43(3):230-233.
- Soboroff SH, Pappius EM, Komaroff AL. Benefits, risks, and costs of alternative approaches to the evaluation and treatment of severe ankle sprain. Clin Orthop Relat Res 1984;(183):160-168.
- Verhagen RA, de Keizer G, van Dijk CN. Long-term follow-up of inversion trauma of the ankle. Arch Orthop Trauma Surg 1995;114(2):92-96.
- Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med 2005;39(3):1-4.
- Smith RW, Reischl SF. Treatment of ankle sprains in young athletes. Am J Sports Med 1986;14(6):465-471.
- Peters JW, Trevino SG, Renstrom PA. Chronic lateral ankle instability. Foot Ankle 1991;12(3):182-191.
- Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med 2006;34(4):612-620.
- Davids K, Glazier P. Deconstructing neurobiological coordination: the role of the biomechanics-motor control nexus. Exerc Sport Sci Rev 2010;38(2):86-90.
- McKeon PO, Hertel J. The dynamical-systems approach to studying athletic injury. Athl Ther Today 2006;11(1):31-33.
- Wikstrom EA, Hubbard-Turner T, McKeon PO. Understanding and treating lateral ankle sprains and their consequences: a constraints-based approach. Sports Med 2013;43(6):385-393.
- Beynnon BD, Renstrom PA, Alosa DM, et al. Ankle ligament injury risk factors: a prospective study of college athletes. J Orthop Res 2001;19(2):213-220.
- McKeon PO, Hertel J. Systematic review of postural control and lateral ankle instability, part I: can deficits be detected with instrumented testing. J Athl Train 2008;43(3):293-304.
- Hiller CE, Nightingale EJ, Lin CW, et al. Characteristics of people with recurrent ankle sprains: a systematic review with meta-analysis. Br J Sports Med 2011;45(8):660-672.
- Hoch MC, McKeon PO, Andreatta RD. Plantar vibrotactile detection deficits in adults with chronic ankle instability. Med Sci Sports Exerc 2012;44(4):666-672.
- McKeon JM, McKeon PO. Evaluation of joint position recognition measurement variables associated with chronic ankle instability: a meta-analysis. J Athl Train 2012;47(4):444-456.
- Drewes LK, McKeon PO, Kerrigan DC, Hertel J. Dorsiflexion deficit during jogging with chronic ankle instability. J Sci Med Sport 2009;12(6):685-687.
- Hoch MC, Staton GS, Medina McKeon JM, et al. Dorsiflexion and dynamic postural control deficits are present in those with chronic ankle instability. J Sci Med Sport 2012;15(6):574-579.
- Hoch MC, McKeon PO. Peroneal reaction time after ankle sprain: a systematic review and meta-analysis. Med Sci Sports Exerc 2014;46(3):546-556.
- Arnold BL, De La Motte S, Linens S, Ross SE. Ankle instability is associated with balance impairments: a meta-analysis. Med Sci Sports Exerc 2009;41(5):1048-1062.
- Brown C. Foot clearance in walking and running in individuals with ankle instability. Am J Sports Med 2011;39(8):1769-1776.
- Brown C, Bowser B, Simpson KJ. Movement variability during single leg jump landings in individuals with and without chronic ankle instability. Clin Biomech 2012;27(1):52-63.
- Drewes LK, McKeon PO, Paolini G, et al. Altered ankle kinematics and shank-rear-foot coupling in those with chronic ankle instability. J Sport Rehabil 2009;18(3):375-388.
- Herb CC, Chinn L, Dicharry J, et al. Shank-rearfoot joint coupling with chronic ankle instability. J Appl Biomech 2014;30(3):366-372.
- Monaghan K, Delahunt E, Caulfield B. Ankle function during gait in patients with chronic ankle instability compared to controls. Clin Biomech 2006;21(2):168-174.
- Hoch MC, McKeon PO. Integrating contemporary models of motor control and health in chronic ankle instability. Athl Train Sports Health Care 2010;2(2):82-88.
- Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br 1965;47(4):678-685.
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 2004;91(1):410-423.
- McKeon PO, Booi MJ, Branam B, et al. Lateral ankle ligament anesthesia significantly alters single limb postural control. Gait Posture 2010;32(3):374-377.
- Hoch MC, McKeon PO. Joint mobilization improves spatiotemporal postural control and range of motion in those with chronic ankle instability. J Orthop Res 2011;29(3):326-332.
- McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord 2008;9:76.
- Wikstrom EA, Fournier KA, McKeon PO. Postural control differs between those with and without chronic ankle instability. Gait Posture 2010;32(1):82-86.
- Wikstrom EA, Tillman MD, Borsa PA. Detection of dynamic stability deficits in subjects with functional ankle instability. Med Sci Sports Exerc 2005;37(2):169-175.
- Wikstrom EA, Tillman MD, Chmielewski TL, et al. Dynamic postural stability deficits in subjects with self-reported ankle instability. Med Sci Sports Exerc 2007;39(3):397-402.
- Wikstrom EA, Tillman MD, Chmielewski TL, et al. Dynamic postural control but not mechanical stability differs among those with and without chronic ankle instability. Scand J Med Sci Sports 2010;20(1):e137-144.
- McKeon PO, Ingersoll CD, Kerrigan DC, et al. Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc 2008;40(10):1810-1819.
- McKeon PO, Paolini G, Ingersoll CD, et al. Effects of balance training on gait parameters in patients with chronic ankle instability: a randomized controlled trial. Clin Rehabil 2009;23(7):609-621.
- McKeon PO, Mattacola CG. Interventions for the prevention of first time and recurrent ankle sprains. Clin Sports Med 2008;27(3):371-382.
- Hoch MC, Andreatta RD, Mullineaux DR, et al. Two-week joint mobilization intervention improves self-reported function, range of motion, and dynamic balance in those with chronic ankle instability. J Orthop Res 2012;30(11):1798-1804.
- Hoch MC, McKeon PO. Joint mobilization improves spatiotemporal postural control and range of motion in those with chronic ankle instability. J Orthop Res 2010;29(3):326-332.
- Wikstrom EA, McKeon PO. Manipulative therapy effectiveness following acute lateral ankle sprains: a systematic review. Athl Train Sports Health Care 2011;6(3):271-279.
- LeClaire J, Wikstrom EA. Massage improves postural control in those with chronic ankle instability. Athl Train Sports Health Care 2012;4(5):213-219.
- Vaillant J, Rouland A, Martigne P, et al. Massage and mobilization of the feet and ankles in elderly adults: Effect on clinical balance performance. Man Ther 2009;14(6):661-664.
- Abrahamova D, Mancini M, Hlavacka F, Chiari L. The age-related changes of trunk responses to Achilles tendon vibration. Neurosci Lett 2009;467(3):220-224.
- Capicikova N, Rocchi L, Hlavacka F, et al. Human postural response to lower leg muscle vibration of different duration. Physiol Res 2006;55(Suppl 1):S129-S134.
- Terada M, Pietrosimone BG, Gribble PA. Therapeutic interventions for increasing ankle dorsiflexion after ankle sprain: a systematic review. J Athl Train 2013;48(5):696-709.
- McKeon PO, Medina McKeon JM, Mattacola CG, Lattermann C. Finding context: a new model for interpreting clinical evidence. Int J Athl Ther Train 2011;16(5):10-13.
- Eechaute C, Vaes P, Van Aerschot L, et al. The clinimetric qualities of patient-assessed instruments for measuring chronic ankle instability: a systematic review. BMC Musculoskelet Disord 2007;8:6.
- Docherty CL, Valovich McLeod TC, Shultz SJ. Postural control deficits in participants with functional ankle instability as measured by the balance error scoring system. Clin J Sport Med 2006;16(3):203-208.
- Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med 2010;38(4):829-834.
- Wikstrom EA, Anderson RB. Alterations in gait initiation are present in those with posttraumatic ankle osteoarthritis: a pilot study. J Appl Biomech 2013;29(3):245-252.
- Powell MR, Powden CJ, Houston MN, Hoch MC. Plantar cutaneous sensitivity and balance in individuals with and without chronic ankle instability. Clin J Sport Med 2014;24(6):490-496.









Could we perhaps be overthinking this sensorimotor theory. Maybe the talocrural joint mobilization and calf stretching simply helps to restore lost DF mobility which in turn allows better (more efficient) functioning of the ankle stabilizers. Then the intrinsic soft tissue mobilization simply frees up the reflexive spasming and overuse spasms as a result of the injury and altered gait following the injury. I know physical therapy as a profession is trying to be more “scientific” but also need to be honest with ourselves as to the value of our research. Why aren’t PTs doing talocrural mobs and calf stretching and intrinsic soft tissue work already? As a PT, I would be hard pressed to find a patient who suffered a significant, much less multiple ankle sprains who presented with unremarkable ankle DF, or soft and supple intrinsics, or a flexible and unrestricted calf. I don’t mean to be cynical, but let’s be honest about what we’re doing.