When preventive measures have been exhausted, various surgical approaches can be employed to provide each diabetic patient with a functional, biomechanically sound foot that is free of infection, while minimizing the risk of future lower limb complications.
When preventive measures have been exhausted, various surgical approaches can be employed to provide each diabetic patient with a functional, biomechanically sound foot that is free of infection, while minimizing the risk of future lower limb complications.
By Gabriel V. Gambardella, DPM, and Peter A. Blume, DPM, FACFAS
Diabetic foot syndrome is one of the most common long-term complications of chronic hyperglycemia and a major source of morbidity and mortality. More than 60% of nontraumatic amputations are attributed to diabetes, 80% of which are preceded by a foot ulcer.1
Peripheral neuropathy, peripheral vascular disease, ipsilateral foot ulcer, previous amputation, male gender, and insulin therapy have been reported as risk factors for lower extremity amputation (LEA).2,3 Limb preservation in patients with diabetes mellitus is a challenging process that aims to prevent major limb loss in a population often plagued by multiple comorbidities. Employing a multidisciplinary approach has proved advantageous in improving limb salvage rates in the diabetic population.4
Prevention of diabetic foot disease through glycemic control, periodic foot examinations, callus debridement, shoe-gear recommendations, deformity prevention and accommodation, and patient education is the first line of defense against amputation. However, surgical intervention frequently becomes necessary to eradicate infection, remove necrotic tissue, close chronic wounds, eliminate structural causes of tissue breakdown, and reconstruct deformities.
The foot and ankle surgeon is one of the foremost players in the limb salvage effort, assuming the difficult tasks of preserving extremities through various surgical techniques and preventing and managing complications postoperatively. Providing each patient with a functional, biomechanically sound, and shoe-able foot that is free of infection, while minimizing the risk of tissue breakdown and recurrent infection, is integral to a successfully salvaged limb. Efficiently achieving these objectives decreases the threat of a major LEA, prolongs survival, promotes independence, and evades quality of life (QOL) deterioration, as will be explained in detail below.

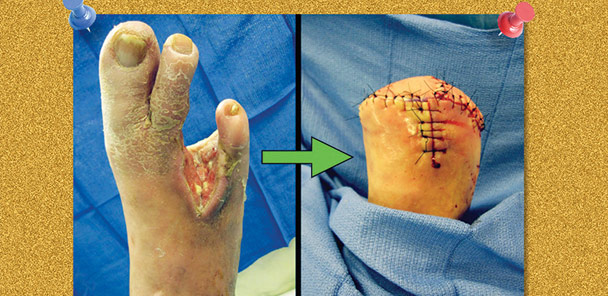
Figure 1. A biomechanically compromised foot secondary to extensive bone resection and soft tissue debridement following a diabetic foot infection. The patient required a transmetatarsal amputation.
Why choose limb salvage?
For a myriad of reasons, limb salvage should be considered a first-line approach in treating most patients with an at-risk limb. It has been demonstrated that five-year mortality rates are higher in those with a diabetes-related LEA than those afflicted with breast cancer or prostate cancer.5
Following below- and above-knee amputations, 30-day mortality rates of 6.3% and 13.3%, respectively,6 have been reported. Long-term survival rates have been documented as low as 62% at one year, 50% at two years, and 29% at five years.7 In diabetic patients with end-stage renal disease (ESRD) on hemodialysis, the one-year mortality rate approaches almost 50%.8 Conversely, significantly better mortality rates have been reported following partial foot amputations,9,10 which also spare patients the need for intensive rehabilitation. While increased energy expenditure and oxygen consumption during prosthetic gait11,12 in patients with transtibial and transfemoral amputations have been observed and considered a disadvantage of proximal amputation, other studies have been unable to find a statistically significant difference when compared to partial foot amputees.13
Furthermore, major limb loss in older, diabetic, and dysvascular patients has been associated with a deteriorating QOL. Many amputees are unable to achieve premorbid levels of ambulatory mobility, remain confined to their homes if without assistance, and require a wheelchair for mobilization.14-16 They experience considerably more problems than age- and sex-matched controls with performing household chores and hobbies and with maintaining social relationships due to their inability to be effectively rehabilitated into the community.14 Psychosocial effects,17 as well as a moderate risk for loss of the contralateral limb,18,19 are also concerns following LEA.

Figure 2. Bilateral gangrene of the distal aspect of the pedal digits. Partial pandigital amputations were performed to maximize foot length.
Similarly, diabetic foot ulcers can have a profound effect on QOL if not treated appropriately. Healing index wounds has been shown to preserve QOL,20 whereas those who fail to heal, or who develop recurrent ulcers, have reported deterioration on the physical functioning, general health, and mental health QOL domains of the Short Form-36 assessment tool.20 Ribu and colleagues demonstrated that QOL improved substantially for the social functioning and mental health domains of the SF-36 as diabetic foot ulcers healed.21 However, studies have revealed alarmingly high ulcer recurrence rates,22-24 translating into continued office visits for serial debridements, wound reconstruction, and a prolonged modified ambulatory status. Plantar ulcer location, osteomyelitis, HbA1C levels of more than 7.5%, and elevated C-reactive protein levels have been associated with ulcer recurrence in the diabetic population.23
If wounds fail to heal due to compromised perfusion, become infected, or result in osteomyelitis, a minor amputation can be considered. Following partial foot amputation (Figure 1), patients exhibit fewer physical limitations than those with a chronic foot wound or transtibial amputation.25 However, with any pedal amputation, altered biomechanics must be addressed to prevent pathological gait patterns and tissue breakdown under areas of increased pressure.
Determining the best option for the patient
Limb salvage can be a time-consuming process fraught with complications and costs. A comprehensive discussion between the surgeon and patient is critical for aligning realistic expectations in terms of postoperative function, healing potential, and the progressive nature of the underlying disease.
The patient must understand the continued risk of tissue breakdown, healing complications, and recurrent infections that could require additional hospitalizations, surgical procedures, and perhaps a major LEA. Advanced wound closure techniques, deformity reconstruction, or subsequent pedal amputations may be necessary to achieve a successful outcome.
Four pertinent issues must be addressed with the limb-salvage candidate prior to surgical intervention:26
- Definition of a realistic expectation, corresponding to the average functional outcome in medically similar patients with the same amputation level. This is not considered to be the best possible outcome;
- Comparison of the functional outcomes of a salvaged limb versus an amputation and fitting of a prosthesis;
- Costs, including resource consumption, time off from work, and the patient’s ability to afford multiple surgeries; and
- Risks, particularly risks of recurrent infection and the need for further surgery when limb salvage is chosen.
A thorough vascular examination, including Doppler ultrasound, is essential for determining perfusion adequacy of the corresponding angiosomes. When perfusion is impaired, patients should have a formal consultation with a vascular surgeon to determine the extent of vascular disease and the need for vascular reconstruction.
An angiogram can establish the need for revascularization of the posterior tibial, anterior tibial, peroneal, or pedal arteries through either arterial bypass surgery or endovascular techniques. Although direct revascularization (revascularization of an angiosome through the artery by which it is directly perfused) is preferred so as not to rely on interarterial connections between angiosomes, an indirect revascularization can also render acceptable limb salvage rates.27
Faglia and colleagues found that more than 96% of diabetic patients with critical limb ischemia can be revascularized using either percutaneous transluminal angioplasty or bypass graft surgery.28 Taylor and Porter reported a 74% long-term limb salvage rate in severely ischemic limbs that underwent emergent surgical treatment of a diabetic foot infection, gangrene, or infected neurotrophic ulcer when combined with lower extremity revascularization, and recommended that major LEAs be considered only as a last resort in diabetic patients with a foot infection or localized gangrene.29
The vascular exam and postreperfusion results should provide sufficient information to determine the ideal amputation level. If revascularization is not possible, or fails to restore adequate pedal hemodynamics, a primary below-knee or above-knee amputation should be offered to the patient.
In addition to critical limb ischemia in a nonreperfusable foot, there are a number of other scenarios in which a below-knee or above-knee amputation becomes the best option for the patient. A nonsalvageable foot secondary to extensive tissue loss, unstable deformity in a poor surgical candidate, and nonambulatory bedridden patients who likely suffer from extensive comorbid conditions30 are indications for a primary major LEA where the benefit of a more definitive surgery outweighs the risks of attempted limb salvage, its potential complications, and a poor prognosis.
Assessing the patient’s premorbid functional status, postoperative ambulatory expectations, and life expectancy further aids in decision-making. Elderly patients may have the most to gain from limb salvage if it enables them to function independently, as many patients whose preoperative activity is limited and who are medically compromised cannot be fully rehabilitated following major limb loss.31,32 Younger, healthier, active patients may be more functional with a major amputation and prosthesis rather than a salvaged, yet biomechanically compromised, extremity that precludes them from participating in certain physical activities.31
Calciphylaxis, or calcific uremic arteriolopathy, is a rare syndrome most commonly encountered in patients with ESRD on hemodialysis and secondary hyperparathyroidism. The life-threatening vasculopathy, diagnosed using tissue biopsy and histological analysis, presents clinically as cutaneous necrosis, nonhealing ulcers, calcified subcutaneous nodules, and gangrene.33,34 It is associated with a high mortality rate, typically due to overwhelming sepsis,35,36 and is a poor prognostic indicator for limb salvage surgery.34 Treatment should focus on infection prevention and pain management. We maintain that these patients are poor candidates for limb salvage.

Figure 3. Closure of a chronic hindfoot wound utilizing a reverse sural artery flap. A split-thickness skin graft was harvested from the ipsilateral leg and applied to the donor site.
Surgical aspects of limb salvage
Patients requiring surgical intervention will demonstrate particular findings upon clinical examination,37 including a pedal wound recalcitrant to local wound care, infected ulcer, gangrene, deep space abscess, deformity, or suspected osteomyelitis. Each diagnosis warrants a different route of limb salvage, which may include:
- Incision and drainage of an abscess;
- Debridement of infected and necrotic soft tissue and bone;
- Resection of prominent osseous structures precipitating skin breakdown;
- Amputation of an ischemic or infected aspect of the foot (Figure 2);
- Wound reconstruction utilizing pedicled or free flaps and skin grafts (Figure 3); and
- Deformity reconstruction.
Although each pursuit relates to a particular indication, the goal remains the same: to maximize function by providing the patient with a stable infection-free foot, while minimizing the risk of tissue breakdown, healing complications, reinfection, and the need for a proximal amputation.
Charcot deformity
Neuropathic osteoarthropathy, commonly referred to as Charcot foot, is most commonly encountered in patients with diabetes-induced peripheral neuropathy, but is also encountered in patients with neuropathy secondary to other causes, such as drug and alcohol abuse, HIV, tertiary syphilis, syringomyelia, and radiculopathy.38-41
The pathology is radiographically hallmarked by progressive osseous destruction and joint subluxation that manifests clinically as a swollen, red, and hot foot, ankle, or both that somewhat resembles cellulitis, or infection. The pathology results in marked instability and possible midfoot collapse. If healing fails to restore an anatomically acceptable posture following conservative treatment methods, surgical correction becomes necessary to construct a stable plantigrade foot capable of ambulation.
Surgical reconstruction involves excising diseased and infected bone and soft tissue, reducing prominent osseous structures that pose a risk for tissue breakdown, correctional osteotomies, and multiple joint arthrodesis using various methods of fixation, including screws, plates, intramedullary nails, and external fixation devices (Figure 4). Bone grafting can be used to negate the shortening of the leg or foot inherent with the procedure.
Selection criteria and contraindications for reconstruction have been defined.42 Indications include recurrent ulcers or infections in the setting of a stable deformity and unstable deformities that cannot be controlled with a Charcot restraint orthotic walker or other types of bracing and footwear. The patient should demonstrate sufficient arterial perfusion and any underlying osteomyelitis should be treated prior to the final reconstruction.
Prior to surgery, the patient’s treatment should be medically optimized and he or she should have been ambulatory prior to developing the arthropathy, display compliance with the surgeon’s instructions, understand the severity of the condition and the importance of preventing postoperative complications, and have a support system available. The demanding postoperative course requires the patient to maintain a nonweight-bearing status for at least three to four months, or until osseous consolidation becomes evident.

Figure 4. Osteoarthropathy of the rearfoot and ankle. A tibiocalcaneal and midtarsal arthrodesis was performed utilizing external fixation to salvage the limb and create a stable plantigrade foot.
The surgery is not recommended in bedridden patients, those who are likely to be noncompliant, and those with arterial insufficiency in which bone and wound healing are compromised, untreated osteomyelitis, or severe soft tissue loss. We recommend caution be taken in patients with ESRD, morbid obesity, poor nutritional status, psychosocial issues, tobacco use, and venous insufficiency, as these conditions can potentially increase the postoperative complication rate and affect the overall outcome.
Although successful limb salvage has been reported in more than 90% of patients,43-45 complications occur frequently, and the surgeon should anticipate them and be prepared to manage events when they occur. Cited complications following Charcot reconstruction include pin tract infections, malunions, nonunions, superficial and deep infections, osteomyelitis, impaired wound healing, hematomas, hardware failure, and need for revision or LEA.46-49
Conclusion
Diabetic limb salvage surgery employs various surgical techniques designed to prevent major LEA. The surgeon and patient must be prepared to spend a considerable amount of time and effort to achieve a satisfactory result. Because of the unpredictability of surgical outcomes, pathology recurrence, and high rate of postoperative complications, patients are best treated by a surgeon with extensive experience in this aspect of foot and ankle surgery.
Gabriel V. Gambardella, DPM, is chief resident in the Podiatric Medicine and Surgery Residency Program at Yale-New Haven Hospital in New Haven, CT. Peter A. Blume, DPM, FACFAS, is assistant clinical professor in the departments of surgery, anesthesia, and orthopaedics, and rehabilitation at Yale University School of Medicine in New Haven.
- Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 2010;52(3 Suppl):17S-22S.
- Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy and foot ulcers. Diabetes Care 1999;22(7):1029-1035.
- Moura Neto A, Zantut-Wittmann DE, Fernandes TD, et al. Risk factors for ulceration and amputation in diabetic foot: study in a cohort of 496 patients. Endocrine 2013;44(1):119-124.
- Zayed H, Halawa M, Maillardet L, et al. Improving limb salvage rate in diabetic patients with critical leg ischaemia using a multidisciplinary approach. Int J Clin Pract 2009;62(6):855-858.
- Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007;4(4):286-287.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veteran Affairs National Surgical Quality Improvement Program. Surgery 2001;130(1):21-29.
- Ploeg AJ, Lardenoye JW, Vrancken Peeters MP, Breslau PJ. Contemporary series of morbidity and mortality after lower limb amputation. Eur J Vasc Endovasc Surg 2005;29(6):633-637.
- Lavery LA, Hunt NA, Ndip A, et al. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010;33(11):2365-2369.
- Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg 2006;45(2):91-97.
- McKittrick LS, McKittrick JB, Risley TS. Transmetatarsal amputation for infection or gangrene in patients with diabetes mellitus. Ann Surg 1949;130(4):826-840.
- Huang CT, Jackson JR, Moore NB, et al. Amputation: energy cost of ambulation. Phys Med Rehabil 1979;60(1):18-24.
- Genin JJ, Bastien GJ, Franck B, et al. Effect of speed on the energy cost of walking in unilateral traumatic lower limb amputees. Eur J Appl Physiol 2008;103(6):655-663.
- Goktepe AS, Cakir B, Yilmaz B, Yazcioglu K. Energy expenditure of walking with prostheses: comparison of three amputation levels. Prosthet Orthot Int 2010;34(1):31-36.
- Pell JP, Donnan PT, Fowkes FG, Ruckley CV. Quality of life following lower limb amputation for peripheral arterial disease. Eur J Vasc Surg 1993;7(4):448-451.
- Peng CW, Tan SG. Perioperative and rehabilitative outcomes after amputation for ischaemic leg gangrene. Ann Acad Med Singapore 2000;29(2):168-172.
- Czerniecki JM, Turner AP, Williams RM, et al. Mobility changes in individuals with dysvascular amputation from the presurgical period to 12 months postamputation. Arch Phys Med Rehabil 2012;93(10):1766-1773.
- Parkes CM. Psycho-social transitions: comparison between reactions to loss of a limb and loss of a spouse. Br J Psychiatry 1975;127:204-210.
- Bodily KC, Burgess EM. Contralateral limb and patient survival after leg amputation. Am J Surg 1983;146(2):280-282.
- Kihn RB, Warren R, Beebe GW. The “geriatric” amputee. Ann Surg 1972;176(3):305-314.
- Winkley K, Stahl D, Chalder T, et al. Quality of life in people with their first diabetic foot ulcer: a prospective cohort study. J Am Podiatr Med Assoc 2009;99(5):406-414.
- Ribu L, Birkeland K, Hanestad BR, et al. A longitudinal study of patients with diabetes and foot ulcers and their health-related quality of life: wound healing and quality-of-life changes. J Diabetes Complications 2008;22(6):400-407.
- Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med 1993;233(6):485-491.
- Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis of a Eurodiale subgroup. Int Wound J 2012 Jun 19. [Epub ahead of print]
- Kloos C, Hagen F, Lindloh C, et al. Cognitive function is not associated with recurrent foot ulcers in patients with diabetes and neuropathy. Diabetes Care 2009;32(5):894-896.
- Boutoille D, Féraille A, Maulaz D, Krempf M. Quality of life with diabetes-associated foot complications: comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int 2008;29(11):1074-1078.
- Pinzur MS, Gottschalk FA, Pinto MA, Smith DG. Controversies in lower-extremity amputation. J Bone Joint Surg Am 2007;89(5):1118-1127.
- Kabra A, Suresh KR, Vivekenand V, et al. Outcomes of angiosome and non-angiosome targeted revascularization in critical lower limb ischemia. J Vasc Surg 2013;57(1):44-49.
- Faglia E, Clerici G, Losa S, et al. Limb revascularization feasibility in diabetic patients with critical limb ischemia: results from a cohort of 344 consecutive unselected diabetic patients evaluated in 2009. Diabetes Res Clin Pract 2012;95(3):364-371.
- Taylor LM Jr, Porter JM. The clinical course of diabetics who require emergent foot surgery because of infection or ischemia. J Vasc Surg 1987;6(5):454-459.
- Wukich DK, Pearson KT. Self-reported outcomes of trans-tibial amputations for non-reconstructable Charcot neuroarthropathy in patients with diabetes: a preliminary report. Diabet Med 2013;30(3):387-390.
- Brown BJ, Crone CG, Attinger CE. Amputation in the diabetic to maximize function. Semin Vasc Surg 2012;25(2):115-121.
- Buzato MA, Tribulatto EC, Costa SM, et al. Major amputations of the lower leg. The patients two years later. Acta Chir Belg 2002;102(4):248-252.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol 1999;40(6 Pt 1):979-987.
- Mureebe L, Moy M, Balfour E, et al. Calciphylaxis: a poor prognostic indicator for limb salvage. J Vasc Surg 2001;33(6):1275-1279.
- Roe SM, Graham LD, Brock WB, Barker DE. Calciphylaxis: early recognition and management. Am Surg 1994;60(2):81-86.
- Duh QY, Lim RC, Clark OH. Calciphylaxis in secondary hyperparathyroidism. Diagnosis and parathyroidectomy. Arch Surg 1991;126(10):1213-1218.
- Gambardella GV, Blume PA. Understanding the biomechanics of the transmetatarsal amputation. Podiatry Today 2013;26(3):46-56.
- Osterhoff G, Böni T, Berli M. Recurrence of acute charcot neuropathic osteoarthropathy after conservative treatment. Foot Ankle Int 2013;34(3):359-364.
- Classen JN. Neurotrophic arthropathy with ulceration. Ann Surg 1964;159(6):891-894.
- Deng X, Wu L, Yang C, Xu Y. Neuropathic arthropathy caused by syringomyelia. J Neurosurg Spine 2013;18(3):303-309.
- Young N, Neiderer K, Martin B, et al. HIV neuropathy induced Charcot neuroarthropathy: a case discussion. Foot 2012;22(3):112-116.
- Zgonis T, Stapleton JJ, Jeffries LC, et al. Surgical treatment of Charcot neuroarthropathy. AORN J 2008;87(5):971-986.
- Grant WP, Garcia-Levin SE, Sabo RT, et al. A retrospective analysis of 50 consecutive Charcot diabetic salvage reconstructions. J Foot Ankle Surg 2009;48(1):30-38.
- Pinzur MS, Gil J, Belmares J. Treatment of osteomyelitis in charcot foot with single-stage resection of infection, correction of deformity, and maintenance with ring fixation. Foot Ankle Int 2012;33(12):1069-1074.
- Dalla Paola L, Volpe A, Varotto D, et al. Use of a retrograde nail for ankle arthrodesis in Charcot neuroarthropathy: a limb salvage procedure. Foot Ankle Int 2007;28(9):967-970.
- Herscovici D, Sammarco GJ, Sammarco VJ, Scaduto JM. Pantalar arthrodesis for post-traumatic arthritis and diabetic neuroarthropathy of the ankle and hindfoot. Foot Ankle Int 2011;32(6):581-588.
- Mittlmeier T, Klaue K, Haar P, Beck M. Should one consider primary surgical reconstruction in charcot arthropathy of the feet? Clin Orthop Relat Res 2010;468(4):1002-1011.
- Zarutsky E, Rush SM, Schuberth JM. The use of circular wire external fixation in the treatment of salvage ankle arthrodesis. J Foot Ankle Surg 2005;44(1):22-31.
- Mendicino RW, Catanzariti AR, Saltrick KR, et al. Tibiotalocalcaneal arthrodesis with retrograde intramedullary nailing. J Foot Ankle Surg 2004;43(2):82-86.