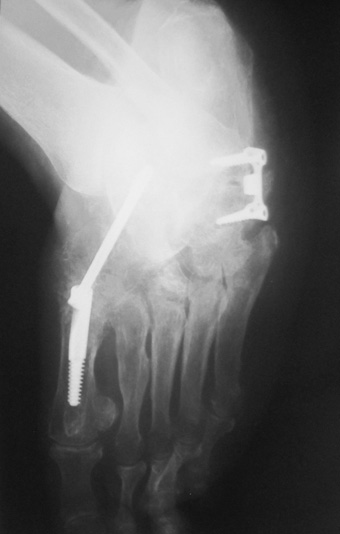
Research indicates that in diabetic patients with Charcot neuroarthropathy, peripheral bone mineral density decreases over time, which can contribute to risks of hardware failure, loss of correction, delayed union, and nonunion in patients with advanced disease.
By Rachel H. Jung, DPM, MHA, MPH; Robert M. Greenhagen, DPM; Dane K. Wukich, MD; Vassilios Vardaxis, PhD; and Robert M. Yoho, DPM, MS
Estimates suggest more than 200 million individuals around the world currently are affected by diabetes mellitus (DM), a statistic that is projected to double in number by the year 2030.1 In developed countries, approximately 1 in 1000 diabetic patients have Charcot neuroarthropathy (CN), a neuromusculoskeletal condition. The statistics also show that nearly 30% of diabetic patients with peripheral neuropathy are affected by CN.2
DM is a multisystemic disease that can have potentially profound effects on the musculoskeletal system, as manifested by conditions such as CN. CN occurs in diabetic patients with peripheral neuropathy, which impedes protection and recovery from cumulative and repetitive lower extremity musculoskeletal trauma. This leads to a cascade of events of further injury and deformity in which patients are prone to debilitation and even premature mortality.3,4
CN is a progressive osseous disease that presents a great challenge to clinicians due to the difficulty of early recognition and the complex management process. However, clinicians can detect the disease through serial radiographic findings and use those findings as a basis for disease classification under the Eichenholtz system. Stage I, the developmental stage, demonstrates subchondral fragmentation, joint dislocations, fractures, and debris formation.5 Eichenholtz also described osteopenia as one of the earliest osseous findings, and this may be the only radiographic finding if the CN process is diagnosed prior to the onset of bony destruction.5 Stage II, the coalescent stage, is distinguished from stage I by the absorption of the fine debris, fusion of the larger fragments, and sclerosis of the bone ends. Stage III, the reconstructive stage, is characterized by the rounded appearance of the bone ends and major bone fragments, signifying radiographic healing.
With the increasing incidence of diabetes, more clinicians will encounter patients with CN in their practices. As clinicians attempt to identify and manage this catastrophic condition, especially through surgical intervention, it is advisable to carefully assess and consider patients’ changes in bone mineral density (BMD) as measuring bone quality can help predict the prognosis of CN.
BMD and CN study

Figure 1. T-score at the core (lumbar) and the periphery (calcaneal) sites for the control and the two diabetic groups.
The authors designed and conducted a prospective study6 to compare peripheral (calcaneal) and core (lumbar spine, L1-L4) bone densities and evaluate bone quality in a population of patients with and without DM. Those patients with DM were further categorized into subgroups of those with and without CN. An additional aim of this study was examining recovery of BMD in relation to time of onset of the CN event. Its final purpose was investigating the potential role of gender, age, and serum vitamin D on the osseous recovery.
Thirty-three individuals (19 women) participated in the study after approval was granted from the Institutional Review Board of our university, University of Des Moines in Iowa. Patients presenting for foot and ankle care at Des Moines University Foot & Ankle were prescreened for study participation over a three-month period and assigned to one of three groups: control, DM without CN, and DM with reconstructive stage (Stage III) CN.
Individuals in the control group had no medical history of DM, peripheral neuropathy, or metabolic bone disease. Patients with DM but without CN were required to have a clinical diagnosis of DM from a primary care provider. Since 80% of CN patients have a history of diabetes longer than 10 years, only patients with a DM history at least that long were included in the study.7 Diabetic patients without CN had varying degrees of peripheral sensory neuropathy, ranging from full sensation to significant loss of protective sensation as measured by a 10-g Semmes-Weinstein monofilament.

Figure 2. Calcaneal bone mineral density (BMD) relative to estimated time since onset of Charcot neuroarthropathy (CN) in male and female patients.
Inclusion criteria for the CN group included a diagnosis of unilateral stage III CN that had been treated nonoperatively by offloading the extremity with bracing or casting. The diagnosis of CN was made based on history, physical examination, and radiographic findings. All 10 patients demonstrated loss of protective sensation as determined by Semmes-Weinstein 10-g monofilament. Radiographic findings included bone fragmentation, dissolution, joint destruction, and loss of foot architecture not caused by acute trauma or other medical conditions. Patients who met the study criteria were informed of the goals, risks, methods, and purpose of the study and were asked for informed consent.
Bone density scans of the calcaneus and lumbar spine were performed on each participant. Peripheral bone density was measured at the calcaneus using peripheral instantaneous x-ray imaging (PIXI) and was expressed as T-scores and BMD. (T-scores reflect the number of standard deviations above/below the mean for a healthy 30-year-old adult of the same sex and ethnicity as the patient.) PIXI testing was performed on the extremity affected by CN. The investigators elected not to test the contralateral (unaffected) limb due to evidence demonstrating a significant difference in bone density between affected and unaffected limbs.8-11 Previous studies have also shown that BMD in the unaffected limb does not decrease with time.8,10
The core bone density was measured at the lumbar spine L1-L4 using dual-energy x-ray absorptiometry (DEXA) and was also measured as T-scores and BMD. Reference ranges for the T-scores for both scans were defined as: normal (-0.6 and above), osteopenia (-0.7 to -1.6) and osteoporosis (-1.7 and below).12,13 PIXI and DEXA are accepted techniques for evaluating peripheral and axial bone densities.12,13 The World Health Organization recommends central DEXA as the ideal method of studying bone density, while peripheral imaging devices such as PIXI are widely accepted for their ability to detect osteoporosis in peripheral specific regions of the skeleton.13 Peripheral imaging sources such as PIXI are frequently used in bone density studies due to their cost effectiveness, accessibility, minimal radiation exposure, and site-specific nature.
Results
From this study, we were able to obtain several valuable pieces of information regarding the BMD characteristics of diabetic patients with and without CN that may raise awareness among clinicians.
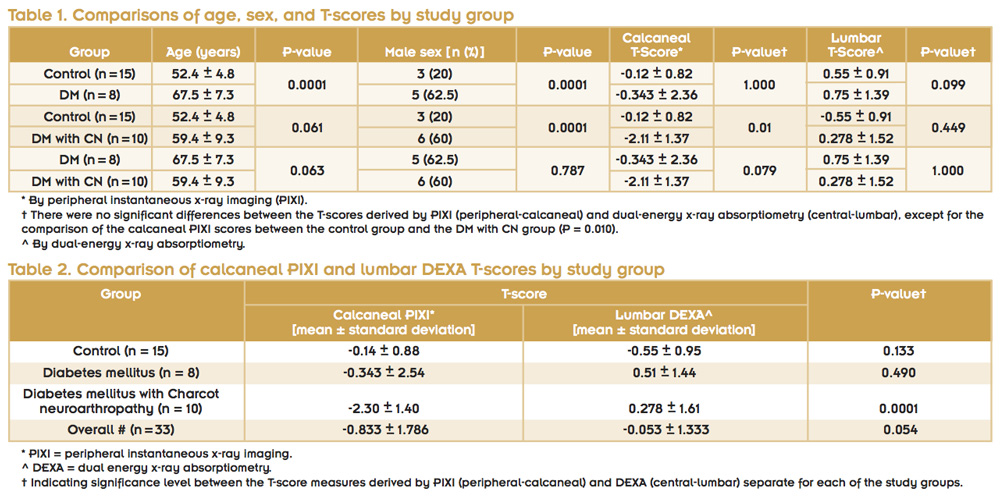
First, we analyzed the differences in bone densities between patients with DM and individuals without DM, and further, between DM patients with CN and without CN (Figure 1, tables 1 and 2). Other studies showed that nondiabetic individuals are prone to undergo a greater amount of axial skeleton demineralization with age than diabetic persons, which is thought to be correlated with hormonal changes as well as the aging process.14,15
In our study, diabetic patients with CN showed lower peripheral BMD of their affected limb compared with their core BMD during the reconstructive stage (Stage III). This finding was observed only in diabetic patients with CN and not in the other two groups; it shows patients with DM, particularly with CN, undergo a localized peripheral osseous demineralization unlike nondiabetic patients. This finding suggests that peripheral BMD of the affected limb assessment is more relevant than BMD of the axial spine for treating lower extremity pathologies like CN. These findings are supported by those of Jirkovská et al, who concluded that the calcaneal BMD of acute Charcot patients is lower than BMD of the spine or femoral neck, whereas calcaneal BMD of nondiabetic controls is greater than BMD of the spine and femoral neck.9 Jirkovská’s study concluded that the central/core BMD of the diabetic patients with CN may represent overall bone quality, but may not accurately reflect the status of BMD in the affected lower extremity.

Figure 4. Calcaneal BMD relative to estimated time since onset of CN in patients younger and older than 60 years.
Additionally, our data show that, for the patients with CN, BMD decreases with time after osseous injuries. Similarly, Petrova and colleagues reported an average calcaneal BMD in the affected limb of .456 g/cm2 compared with .494 g/cm2 (p = 0.001) in the unaffected limb.10 Investigators rescanned patients after three months of immobilization of the affected limb; authors found a statistically significant decrease in BMD occurred only in the affected limb with the average calcaneal density equaling .433 g/cm2 and .482 g/cm2, respectively (p > 0.01).10 A final scan performed after resolution of CN (average, 8.2 months) demonstrated a persistent calcaneal BMD deficit (.432 g/cm2 and .479 g/cm2).10 The affected limbs of these patients were immobilized for three to six months prior to returning to activity.
Lida et al also reported increases in BMD of patients with intertrochanteric hip fractures during the first three years of their injuries, but observed BMD decline after three years.16 The theory behind this phenomenon is most likely due to the conservative treatment regimen of CN that consists of immobilization and use of assistive devices that result in stress shielding, increasing bone loss, and decreasing BMD.
Finally, our study also investigated the role that gender, age, and serum vitamin D have on bone quality. In general, we found that BMD does not improve with time after a CN event (Figure 2). However, when genders were analyzed separately, we found that men have significantly higher BMD than women in stage III CN, but men progressively lost greater amounts of BMD over time compared with women. We also found that all female patients were osteoporotic, but we noted an increase in BMD as time from the initial event increased (Figure 2). Christensen and colleagues reported similar findings in their study of 49 individuals with DM and various health states, including no comorbidities, neuropathy, history of hallux amputation, acute CN, and chronic CN.8 They found that women had lower BMD than men.8
Our data showed calcaneal BMD decreased as participant age increased (Figure 3, Table 3). Our study also evaluated the effect of time since CN onset on calcaneal BMD in subgroups of individuals younger and older than 60 years. We found that patients older than 60 years have lower BMD than patients younger than 60 years, but both groups demonstrated decreases in BMD as time from CN onset increased. However, the difference in BMD was not significantly different between patients younger and older than 60 years (Figure 4).
We also evaluated the effect of serum vitamin D levels on calcaneal BMD. The intimate relationship between vitamin D and skeletal health is well known—specifically that the active form of vitamin D, calcitriol, is required for intestinal absorption of calcium.17 Consistent with this, research has shown serum vitamin D levels are low in diabetic patients with CN.18 Similarly, in our study, all CN patients were vitamin D deficient (Figure 5). However, our gender-based analysis of the CN group revealed that in the men, increased calcaneal BMD was associated with a decrease in serum vitamin D levels, while in the women increased calcaneal BMD was associated with increased serum vitamin D levels. Therefore, vitamin D levels were not shown to be a significant predictor of BMD recovery after a CN event except in women (Figure 5). Despite this finding, we recommend routine tests of vitamin D levels and appropriate management of deficiencies for ideal osseous recovery during and after a CN event.
Conclusion
Findings from our study provide critical information about the unique BMD characteristics of diabetic patients with CN. Axial BMD can serve as a general indicator of a diabetic patient’s overall bone quality. However, peripheral BMD should be evaluated to understand the pathology and help develop treatment plans when dealing with lower extremity conditions such as CN in diabetic patients. This consideration is especially imperative when clinicians are surgically managing lower extremity deformities resulting from CN.19,20
We demonstrated that peripheral BMD decreases as time from CN elapses. We also showed that gender, age, and serum vitamin D levels have various effects on BMD in diabetic patients with CN. As a result, patients with stage III CN have a great propensity for hardware failure, loss of correction, delayed union, and nonunion,21-25 complications we believe are associated with low BMD. To avoid such undesirable outcomes, utilization of locking plates or intramedullary nails can be considered.26-32 We hope the findings from this study will encourage practitioners to evaluate bone quality and consider all the aspects of BMD when providing care for diabetic patients with CN.
Rachel H. Jung, DPM, MHA, MPH, is a fellow at Jewish Hospital & St. Mary’s Healthcare and the University of Louisville Hospital Podiatric Services, Department of Orthopedics, in Louisville, KY. Robert M. Greenhagen, DPM, is in private practice at the Foot and Ankle Center of Nebraska in Omaha. Dane K. Wukich, MD, is associate professor of orthopedic surgery and chief of the Foot and Ankle Division in the Department of Orthopedic Surgery at University of Pittsburgh Medical Center. Vassilios Vardaxis, PhD, is professor of physical therapy at Des Moines University in Iowa. Robert M. Yoho, DPM, MS, is dean of the College of Podiatric Medicine and Surgery at Des Moines University.
Acknowledgment: Portions of this article were adapted from reference 6.
1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047-1053.
2. Herbst SA, Jones KB, Saltzman CL. Pattern of diabetic neuropathic arthropathy associated with the peripheral bone mineral density. J Bone Joint Surg Br 2004;86(3):378-383.
3. Childs M, Armstrong DG, Edelson GW. Is Charcot arthropathy a late sequela of osteoporosis in patients with diabetes mellitus? J Foot Ankle Surg 1998;37(5):437-439.
4. van Baal J, Hubbard R, Game F, Jeffcoate W. Mortality associated with acute charcot foot and neuropathic foot ulceration. Diabetes Care 2010;33(5):1086-1089.
5. Eichenholtz SN. Charcot Joints. Springfield, IL: Charles C. Thomas; 1966.
6. Greenhagen RM, Wukich DK, Jung RH, et al. Peripheral and central bone mineral density in Charcot’s neuroarthropathy compared in diabetic and nondiabetic populations. J Am Podiatr Med Assoc 2012;102(3):213-222.
7. Frykberg RG, ed. The High Risk Foot in Diabetes Mellitus. New York: Churchill Livingstone; 1991.
8. Christensen TM, Bülow J, Simonsen L, et al. Bone mineral density in diabetes mellitus patients with and without a Charcot foot. Clin Physiol Funct Imaging 2010;30(2):130-134.
9. Jirkovská A, Kasalický P, Boucek P, et al. Calcaneal ultrasonometry in patients with Charcot osteoarthropathy and its relationship with densitometry in the lumbar spine and femoral neck and with markers of bone turnover. Diabet Med 2001;18(6):495-500.
10. Petrova NL, Edmonds ME. A prospective study of calcaneal bone mineral density in acute Charcot osteoarthropathy. Diabetes Care 2010;33(10):2254-2256.
11. Petrova NL, Foster AV, Edmonds ME. Calcaneal bone mineral density in patients with Charcot neuropathic osteoarthropathy: differences between type 1 and type 2 diabetes. Diabet Med 2005;22(6):756-761.
12. Pérez-Castrillon JL. Pixi bone density and screening of osteoporosis. Maturitas 2006;55(2):200.
13. Harrison EJ, Adams JE. Application of a triage approach to peripheral bone densitometry reduces the requirement for central DXA but is not cost effective. Calcif Tissue Int 2006;79(4):199-206.
14. Riggs BL, Wahner HW, Seeman E, et al. Changes in bone mineral density of the proximal femur and spine with aging: differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest 1982;70(4):716-723.
15. Riggs BL, Wahner HW, Dunn WL, et al. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest 1981;67(2):328-335.
16. Iida Y, Kuroda T, Kitano T, Mizuno K. Dexa-measured bone density changes over time after intertrochanteric hip fractures. Kobe J Med Sci 2000;46(1-2):1-12.
17. Marks RM, Parks BG, Schon LC. Midfoot fusion technique for neuroarthropathic feet: Biomechanical analysis and rationale. Foot Ankle Int 1998;19(8):507-510.
18. Yoho RM, Frerichs J, Dodson NB, et al. A comparison of vitamin D levels in nondiabetic and diabetic patient populations. J Am Podiatr Med Assoc 2009;99(1):35-41.
19. Bevan WP, Tomlinson MP. Radiographic measures as a predictor of ulcer formation in diabetic Charcot midfoot. Foot Ankle Int 2008;29(6):568-573.
20. Bevilacqua NJ, Rogers LC. Surgical management of Charcot midfoot deformities. Clin Podiatr Med Surg 2008;25(1):81-94.
21. Jolly GP, Zgonis T, Polyzois V. External fixation in the management of Charcot neuroarthropathy. Clin Podiatr Med Surg 2003;20(4):741-756.
22. Pinzur MS, Kelikian A. Charcot ankle fusion with a retrograde locked intramedullary nail. Foot Ankle Int 1997;18(11):699-704.
23. Sayner LR, Rosenblum BI. External fixation for Charcot foot reconstruction. Curr Surg 2005;62(6):618-623.
24. Cummings SR, Cosman F, Jamal S, eds. Osteoporosis: An Evidence-Based Guide to Prevention and Management. Philadelphia, PA: American College of Physicians; 2002.
25. Zarutsky E, Rush SM, Schuberth JM. The use of circular wire external fixation in the treatment of salvage ankle arthrodesis. J Foot Ankle Surg 2005;44(1):22-31.
26. Klos K, Gueorguiev B, Schwieger K, et al. Comparison of calcaneal fixation of a retrograde intramedullary nail with a fixed-angle spiral blade versus a fixed-angle screw. Foot Ankle Int 2009;30(12):1212-1218.
27. Ito K, Hungerbühler R, Wahl D, Grass R. Improved intramedullary nail interlocking in osteoporotic bone. J Orthop Trauma 2001;15(3):192-196.
28. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury 2007;38(Suppl 3):S35-S39.
29. Snow M, Thompson G, Turner PG. A mechanical comparison of the locking compression plate (LCP) and the low contact-dynamic compression plate (DCP) in an osteoporotic bone model. J Orthop Trauma 2008;22(2):121-125.
30. Stoffel K, Booth G, Rohrl SM, Kuster M. A comparison of conventional versus locking plates in intraarticular calcaneus fractures: a biomechanical study in human cadavers. Clin Biomech 2007; 22(1):100-105.
31. Kim T, Ayturk UM, Haskell A, et al. Fixation of osteoporotic distal fibula fractures: a biomechanical comparison of locking versus conventional plates. J Foot Ankle Surg 2007;46(1):2-6.
32. Zehnder S, Bledsoe JG, Puryear A. The effects of screw orientation in severely osteoporotic bone: a comparison with locked plating. Clin Biomech 2009;24(7):589-594.