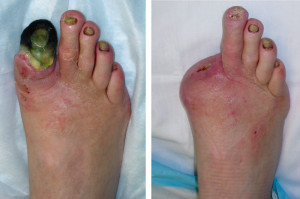
 It’s not uncommon for lower extremity practitioners to encounter undiagnosed peripheral arterial disease in patients who have come in for a seemingly unrelated complaint. An understanding of the risk factors, diagnostic techniques and treatment options is key.
It’s not uncommon for lower extremity practitioners to encounter undiagnosed peripheral arterial disease in patients who have come in for a seemingly unrelated complaint. An understanding of the risk factors, diagnostic techniques and treatment options is key.
By Steven Miller, DPM
Peripheral arterial disease (PAD) encompasses a range of noncoronary arterial syndromes that are caused by alterations in the structure and function of the arteries that supply the brain, visceral organs, and limbs. The clinical manifestations of PAD are a major cause of acute and chronic illness, are associated with decrements in functional capacity and quality of life, cause limb amputation, and increase risk of death. Overall, PAD is increasingly recognized as a health burden worldwide.1
The scope of this article will be limited to disorders of the lower extremity arteries (“lower extremity arterial disease”), which does not include diseases of the aorta, carotid, upper extremity, or visceral arteries. It will focus on vascular diseases caused primarily by atherosclerosis, the most common cause of PAD worldwide, exclusive of aneurysmal, thromboembolic, arteritic, and vasoreactive causes. Presented here are epidemiologic data, diagnostic tests, and therapeutic options.
Prevalence of PAD
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status in the United States using a cross-sectional survey of representative samples of the civilian, noninstitutionalized U.S. population. In the 1999-2000 NHANES survey, 4.3% of U.S. adults aged 40 and older had PAD.2 For those of age 70 or older, PAD was detected in 14.5% of the population. Interestingly, higher values have been found in other countries. PAD has been reported in 18% of Germans of age 65 or older3 and 19% of Dutch individuals of age 55 or older.4 Regardless of geography, the published findings suggest that advancing age is a strong risk factor for PAD.
These three studies primarily defined PAD as having an ankle-brachial index (ABI) below 0.9 in either leg. However, the calculated prevalence of asymptomatic PAD varies significantly according to the ABI assessment method used.5 Interobserver variability of ABI measurement can also be an issue.1 Furthermore, use of ABI as a screening tool has been shown to underreport individuals with arterial calcification.6 It also does not exclude a proximal aneurysm or arterial occlusive disease distal to the ankle.7 Other studies have employed more elaborate inclusion criteria. 8-9
The major cause of lower extremity PAD is atherosclerosis. 1 Cigarette smoking and diabetes mellitus, for example, are exceptionally powerful etiologic risk factors for lower extremity PAD. Other risk factors for atherosclerosis such as dyslipidemia, hypertension, and hyperhomocysteinemia also increase the likelihood of developing lower extremity PAD.1
The systemic nature of the atherosclerotic process also contributes to development of concomitant disease of the arteries serving the heart and brain. Consequently, patients with PAD often face an associated increased risk of cardiovascular ischemic events, such as myocardial infarction (MI), ischemic stroke, and death.1 Studies reveal that in patients with lower extremity PAD:
• 66% have an abnormal stress test.
• Up to 80% have significant disease of at least one coronary artery.
• Risk of heart attack and stroke is increased 20% to 60%.
• Risk of death due to coronary heart disease events is increased two- to sixfold.
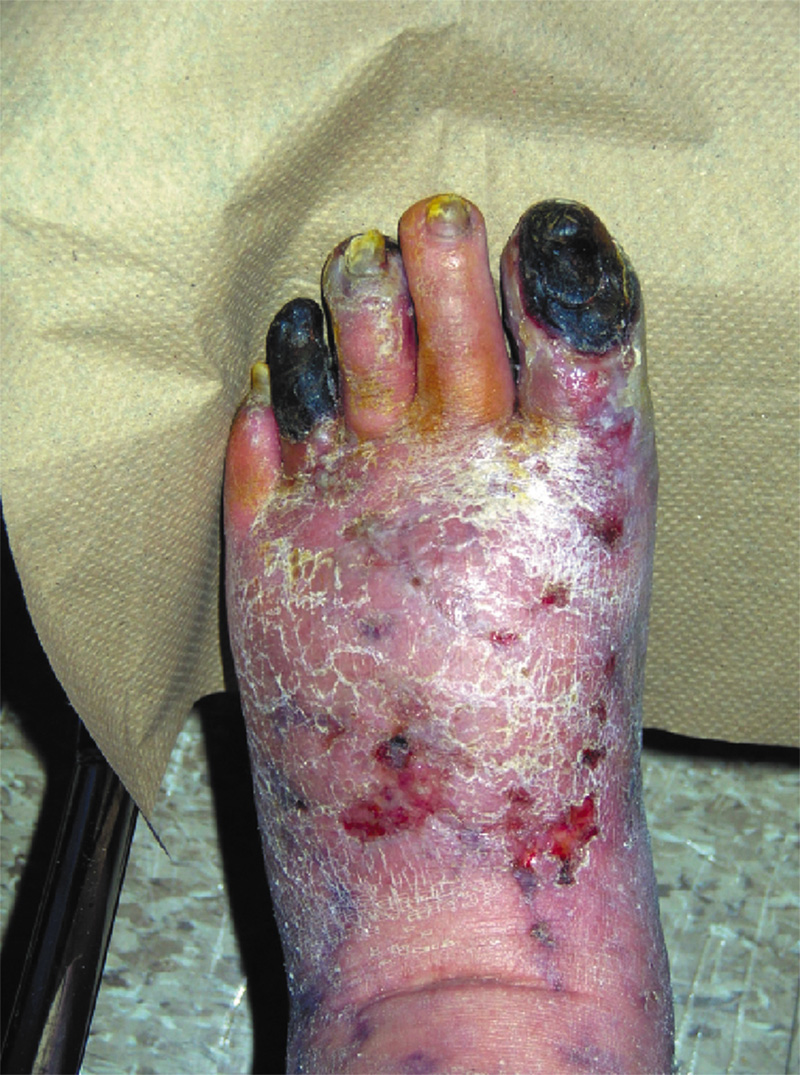
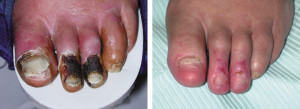
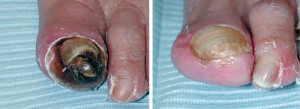
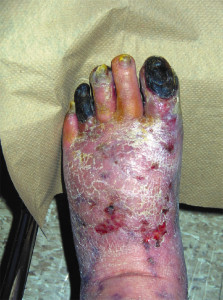
The most severe manifestation of lower extremity peripheral arterial disease, known as critical limb ischemia, is when chronic ischemic rest pain, ulcers or gangrene develop. The one-year mortality rate in patients with critical limb ischemia is approximately 25% and may be as high as 45% in those who have undergone amputation.1
Diagnostic Tests
Every vascular exam of the lower extremity starts with palpating pedal pulses (posterior tibial and dorsalis pedis arteries). When these pulses cannot be palpated, ischemia may be present and further investigation is indicated. Be aware that the dorsalis pedis artery is congenitally absent in 2% of extremities and the posterior tibial artery in 0.1%.6
 Other commonly reported findings in patients with PAD are capillary fill time greater than three seconds and pedal skin that is shiny, atrophic, cool, and hairless. However, independently, these clinical findings are unhelpful in predicting PAD.6
Other commonly reported findings in patients with PAD are capillary fill time greater than three seconds and pedal skin that is shiny, atrophic, cool, and hairless. However, independently, these clinical findings are unhelpful in predicting PAD.6
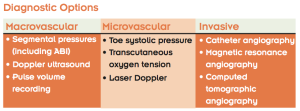
Noninvasive vascular studies are indicated if the outcome will potentially impact the clinical management of the patient. I divide my diagnostic studies into three groups, each containing three tests. The first group evaluates for macroangiopathy, vascular disease affecting visible arteries that are often amenable to surgical or percutaneous procedures. The second group evaluates for microangiopathy. Although these blood vessels are too small to repair in a surgical manner, assessing capillary flow can help predict the likelihood that a skin wound will heal. The final group comprises invasive studies, usually performed prior to surgical or endovascular intervention.
To evaluate the macrovasculature, segmental pressures and ABI are most commonly employed. Segmental pressures refers to the systolic blood pressure measurement taken at four levels on each lower extremity: upper thigh, lower thigh, upper calf, and ankle (posterior tibial and dorsalis pedis arteries). Segmental pressures should be within 30 mmHg of the same level on the contralateral leg or the next level on the same leg. Contraindications for these measurements include excruciating pain in the patient’s legs or feet and the presence of deep venous thrombosis, which could lead to thrombus dislodgment.10
The ABI is calculated by dividing the ankle systolic blood pressure (posterior tibial or dorsalis pedis) by the ipsilateral brachial systolic blood pressure. As noted previously, many factors including arterial calcification and variation in assessments will make the ABI value inaccurate in some patients.
Exercise treadmill tests with measurement of pre-exercise and postexercise ABI values should utilize a standardized protocol with a motorized treadmill.1 These tests may be useful to:
- establish the diagnosis of lower extremity PAD when resting ABI measures are normal,
- objectively document the magnitude of symptom limitation in patients with claudication,
- objectively measure functional improvement in response to claudication treatment,
- differentiate claudication from pseudoclaudication in individuals with exertional leg symptoms, and
- provide objective data to individualize exercise prescriptions in patients with claudication before initiation of a formal program of exercise training.
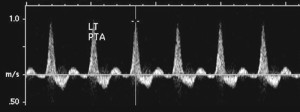
Arterial Doppler is indicated when the ABI is less than 0.9 or arterial calcification is present.1 A waveform of each segment of the vascular tree helps localize any occlusion or stenosis. The waveform should be triphasic, corresponding to the three phases of a heart beat (systole, diastole, elastic recoil). Biphasic waveform indicates mild to moderate disease and monophasic flow indicates significant disease. Duplex scanning can be added to further localize occlusive disease.
Some practitioners choose to perform pulse volume recordings (PVR) instead of or in addition to arterial Doppler. PVR measures change in limb volume between systole and diastole. Any sequential diminution in pulsatility (upstroke and amplitude), a qualitative measurement, signifies the presence of a flow-limiting stenosis in the more proximal arterial segment. Automated equipment has been developed that can provide PVR measurements with ease. Although PVRs are useful and cost-effective, especially as a screening tool, other noninvasive techniques can provide more quantitative perfusion data and better arterial anatomic lower extremity PAD localization.1
A systolic pressure reading of the toe using photoplethysmography or laser Doppler can be a quick and easy tool. This is done with an inflatable toe tourniquet placed around the proximal phalanx with an infrared or laser light placed distally. A toe-brachial index can then be calculated in a similar technique as the ABI is for the ankle, with a normal value equal or greater than 0.7.1
To evaluate for possible isolated islands of hypoperfusion, transcutaneous oxygen tension (TcPO2) is available. TcPO2 determines oxygen diffusion across the skin. A normal value is greater than 30 mmHg.1 It is difficult to get an accurate reading on thick plantar skin, calluses, or in the presence of swelling. Sensors do not stay attached to flaky or moist skin. Because the equipment needs to calibrate before each exam and the patients skin needs to adjust to room temperature, the exam takes approximately 45 minutes.
Invasive studies1 are usually ordered by a vascular specialist. X-ray angiography is the gold standard. Adding digital subtraction provides superior definition by eliminating much of the artifact due to bony structures and dense body tissues. For patients who are unable to tolerate intravenous radiographic contrast media, such as those with an iodine allergy or renal disease, magnetic resonance angiography is typically used. Another option is computed tomographic angiography with nonionic contrast, especially if MR angiography is contraindicated because the patient has a pacemaker, defibrillator, or other metal device implanted in the body.
Treatment
For patients with claudication, the greatest improvement in pain distances (the distance traveled by the patient before onset of claudication pain and the distance to maximal claudication pain) has been associated with exercise interventions of more than 30 minutes per session, at least three sessions per week, with walking as the mode of exercise, use of near-maximal pain during training as the claudication pain end point, and program length of greater than six months.11 Exercise-induced increases in functional capacity and lessening of claudication symptoms may be explained by several mechanisms, including measurable improvements in endothelial vasodilator function, skeletal-muscle metabolism, blood viscosity, and inflammatory responses.12
Pentoxifylline (Trental) and cilostazol (Pletal) have demonstrated benefit to patients with intermittent claudication.13 To prevent blood clots, anti-platelet medication such as aspirin and clopidogrel (Plavix) may be beneficial.13
If the patient is a smoker, smoking cessation should also be incorporated. Clinical trials13,14 have demonstrated that a physician’s advice to stop smoking with a brief three-minute counseling session enhances the rate of smoking cessation. Greatest benefit usually involves counseling with pharmacotherapy. The U.S. Food and Drug Administration has approved seven products for smoking cessation: varenicline, sustained release bupropion and five nicotine-replacement therapies (transdermal patch, gum, lozenge, inhaler, nasal spray). Although nortriptyline and clonidine have not been approved by the FDA as smoking-cessation aids, they have been considered as second-line drugs for smoking cessation.13,14
Studies indicate that intermittent pneumatic compression increases arterial inflow both in patients with stable intermittent claudication and critical limb ischemia.15-19 Furthermore, research suggests that in patients with stable intermittent claudication, long-term benefits such as improvement in walking distance and quality of life as assessed with the Short-Form 36 Health Survey Questionnaire are preserved at one year.15-19 One theory is that intermittent pneumatic compression enhances flow by increasing the arteriovenous pressure gradient.15 Another hypothesis involves the release of nitric oxide, a vasodilator, from endothelium and the development of collateral arteries.20-22
For intermittent pneumatic compression in the treatment of arterial disease, a garment is wrapped around the foot and leg and attached to the pump. When the device is turned on, the foot segment is quickly inflated to 120 mmHg. One second later, the leg segment also inflates to 120 mmHg. They remain inflated for another three seconds, and quickly deflate. A 16 second rest period follows. Thus, the device cycles three times every minute.
Over the past decade, percutaneous revascularization therapies for the treatment of patients with PAD have evolved tremendously, and a great number of patients can now be offered treatment options that are less invasive than traditional surgical options.23 These interventions are usually performed by vascular surgeons, cardiologists and interventional radiologists.24 Compared to vascular surgeons, cardiologists performing lower extremity angioplasty in one study were more likely to treat younger patients with less severe peripheral vascular disease, yet used significantly greater hospital resources (i.e. greater costs) –possibly because peripheral vascular disease is not a cardiologist’s primary focus.25
Balloon angioplasty is utilized to compress an atherosclerotic plaque and stretch a narrowed artery. Its use in the lower extremity is a relatively recent development, and is usually limited to proximal occlusions because of physiologic forces distally.26 Historically, angioplasty for below-knee arterial disease had not been widely accepted because of high restenosis rates, in addition to other possible complications. Inserting a stent appears to reduce the rate of restenosis. Abrupt stent occlusion from extrinsic compression, torsion, and flexion of lower extremity arteries with daily activities27 is of paramount concern, and the same effective pharmacologic algorithm used to prevent abrupt closure after coronary stenting should be employed.28 Be aware that drug-eluting stents require anti-platelet medication, such as those mentioned above, for six to twelve months after insertion to prevent occlusion of the stent. If surgery of any nature is anticipated during this period, a bare metal stent is preferred.
Atherectomy is the method by which atherosclerotic plaques are removed from the artery. A shaver is inserted in the groin (femoral artery) and advanced to the area of narrowing, where the plaque is shaved off and removed. Reports emphasize good primary success and acceptable complication rates.29-32 Atherectomy devices have the distinct advantage of removing the obstructing atherosclerotic or intimal hyperplastic lesions without the disadvantage of introducing a foreign body such as a stent into the artery. If reintervention is required after atherectomy, this can be generally accomplished at the same site with a low risk of complications or discomfort to the patient. Finally, atherectomy also does not preclude the use of bypass for the treatment of peripheral arterial disease nor, in most cases, does it change the anastomotic sites if surgical bypass is required, in contrast to stenting.33 It has taken several different forms, including directional atherectomy, rotational atherectomy, and orbital atherectomy. Laser atherectomy, whereby the plaque is vaporized, also exists, but the number of studies of this technique being applied to PAD is limited.34
The value of spinal cord stimulation in treating lower extremity PAD has been debated. Some have found spinal cord stimulation to be effective in improving microcirculation as measured by TcPO2, reducing pain, and improving limb survival in patients with PAD.35-40 Others dispute these findings, primarily based on a study36-42 performed between 1991 and 1996. In this multi-center randomized prospective study, half of the 120 patients with critical limb ischemia received spinal cord stimulation as part of the treatment protocol. The authors asserted that spinal cord stimulation does not prevent amputation, there are no long-term benefits, and no major improvement in overall pain and quality of life assessment. They concluded that there was insufficient evidence justifying the greater costs of spinal cord stimulation.41-47
Hyperbaric oxygen therapy involves the intermittent inhalation of 100% oxygen in chambers pressurized above 1 atmosphere absolute (ATA), which is the atmospheric pressure at sea level. The benefit of hyperbaric oxygen therapy is based on the premise that raising tissue oxygen levels will enhance wound healing ability. Hyperbaric oxygen therapy is administered either in a monoplace or a multiplace chamber. The chamber is compressed up to 2 to 2.5 ATA while the patient breathes 100% oxygen. Arterial PO2 can increase to 2000 mm Hg.48,49 Most data associated with hyperbaric oxygen therapy for the lower extremities relate to enhancing wound healing in diabetic patients. Some of the benefits are believed to be due to improved tissue oxygenation.50,51 There are few data specific to PAD or arterial ulcers.52
Bypass grafting requires the expertise of a vascular surgeon and is often considered the last option before amputation because of the associated operative mortality.1 Because of the remarkable technological advances, revascularization strategies for many patients have shifted from traditional open surgical approaches toward the percutaneous endovascular treatments described earlier.53
Lower extremity PAD affects millions of Americans, is associated with an increased risk for heart attack and stroke, and can lead to gangrene and amputation. To improve patient and physician awareness of PAD, nationally-recognized published guidelines and recommendations for the diagnosis and treatment are available thanks to the efforts of the American College of Cardiology/American Heart Association task force.1 Development of high-quality diagnostic imaging and less invasive endovascular therapies has increased the number of patients seeking treatment. Health care practitioners must be informed about the risk factors associated with PAD, assessment techniques, and the therapies that are available to combat this disease.
Steven Miller, DPM, is in private practice at Affiliated Podiatrists Ltd. in Chicago, IL.
References
1. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J Am Coll Cardiol 2006;47(6):1239-1312.
2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition examination study, 1999-2000. Circulation 2004;110(6):738-743.
3. Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172(1):95-105.
4. Meijer WT, Hoes AW, Rutgers D, et al. Peripheral arterial disease in the elderly: the Rotterdam study. Arterioscler Thromb Vasc Biol 1998;18(2):185-192.
5. Reed JF 3rd, Eid S, Edris B, Sumner AD. Prevalence of peripheral artery disease varies significantly depending upon the method of calculating ankle brachial index. Eur J Cardiovasc Prev Rehabil 2009;16(3):377-381.
6. McGee SR, Boyko EJ. Physical examination and chronic lower-extremity ischemia: a critical review. Arch Intern Med 1998;158(12):1357-1364.
7. Gaylis H. Diagnosis and treatment of peripheral arterial disease. JAMA 2002;287(3):313.
8. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286(11):1317-1324.
9. Criqui MH, Fronek A, Barrett-Connor E, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71(3):510-515.
10. Grenon SM, Gagnon J, Hsiang Y. Ankle brachial index for assessment of peripheral arterial disease. N Eng J Med 2009;361(19):e40(1-3).
11. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain: A meta-analysis. JAMA 1995;274(12):975-980.
12. Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Eng J Med 2002;347(24):1941-1951.
13. Lesho EP, Manngold J, Gey DC. Management of peripheral arterial disease. Am Fam Phys 2004; 69(3): 525-532.
14. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. May 2008.
15. Labropoulos N, Leon LR, Bhatti A, et al. Hemodynamic effects of intermittent pneumatic compression in patiens with critical limb ischemia. J Vasc Surg 2005;42(4):710-716.
16. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 2005;241(3):431-441.
17. Delis KT, Nicolaides AN, Cheshire NJW, Wolfe JHN. Improvement in walking ability, ankle pressure indices and quality of life in vascular claudication using intermittent pneumatic foot and calf compression: a randomized controlled trial. Br J Surg 2001;88(4):605-606.
18. Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomized controlled trial. Eur J Vasc Surg 2005;30(2):164-175.
19. Ramaswami G, D’Ayala M, Hollier LH, et al. Rapid foot and calf compression increases walking distance in patients with intermittent claudication; results of a randomized study. J Vasc Surg 2005;41(5): 794-801.
20. van Bemmelen PS, Gitlitz DB, Faruqi RM, et al. Limb salvage using high-pressure intermittent compression arterial assist device in cases unsuitable for surgical revascularization. Arch Surg 2001;136(11):1280-1285.
21. van Bemmelen P, Char D, Giron F, Ricotta JJ. Angiographic improvement after rapid intermittent compression treatment [artassist] for small vessel obstruction. Ann Vasc Surg 2003;17(2):224-228.
22. van Bemmelen PS, Mattos MA, Faught WE, et al. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 1994;19(6):1052-1058.
23. Mahmud E, Cavendish JJ, Salami A. Current treatment of peripheral arterial disease: role of percutaneous interventional therapies. J Am Coll Cardiol 2007;50(6):473-490.
24. Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009;50(1):54-60.
25. Vogel TR, Dombrovskiy VY, Haser PB, et al. Lower extremity angioplasty: impact of practitioner specialty and volume on indications and resource utilization. J Vasc Surg 2009;49(5):S1.
26. Bunting TA, Garcia LA. Peripheral atherectomy: a critical review. J Interv Cardiol 2007;20(6):417-424.
27. Rubin BF. Plaque excision in the treatment of peripheral arterial disease. Perspect Vasc Surg Endovasc Ther 2006;18(1):47-52.
28. Feiring AJ, Wesolowski AA, Lade S. Primary stent-supported angioplasty for treatment of below-knee critical limb ischemia and severe claudication: early and one-year outcomes. J Am Coll Cardiol 2004;44(12):2307-2314.
29. Zeller T, Krankenberg H, Steinkamp H, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the multicenter pathway PVD trial. J Endovasc Ther 2009;16(6):653-662.
30. Zeller T, Rastan A, Schwarzwälder U, et al. Midterm results after atherectomy-assisted angioplasty of below-knee arteries with use of the Silverhawk device. J Vasc Interv Radiol 2004;15(12):1391-7.
31. Keeling WB, Shames ML, Stone PA, et al. Plaque excision with the Silverhawk catheter: early results in patients with claudication or critical limb ischemia. J Vasc Surg 2007;45(1):25-31.
32. Stoner MC, deFreitas DJ, Phade SV, et al. Mid-term results with laser atherectomy in the treatment of infrainguinal occlusive disease. J Vasc Surg 2007;46(2):289-295.
33. Shrikhande GV, McKinsey JF. Use and abuse of atherectomy: where should it be used? Semin Vasc Surg 2008;21(4):204-209.
34. Zhou W, Bush RL, Lin PH, et al. Laser atherectomy for lower extremity revascularization: an adjunctive endovascular treatment option. Vasc Endovascular Surg 2006;40(4):268-274.
35. Amann W, Berg P, Gersbach P, et al. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European peripheral vascular disease outcome study. Eur J Vasc Endovasc Surg 2003;26(3)280-286.
36. Tiede JM, Huntoon MA. Review of spinal cord stimulation in peripheral arterial disease. Neuromodulation 2004;7(3):168-175.
37. Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology 2004;55(2):111-118.
38. Ubbink DT, Vermeulen H, Spincemaille GH, et al. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg 2004;91(8):948-955.
39. Ubbink DT, Vermeulen H. Spinal cord stimulation for critical leg ischemia: a review of effectiveness and optimal patient selection. J Pain Symptom Manage 2006;31(4 suppl 1):S30-S35.
40. Gersbach PA, Argitis V, Gardaz JP, et al. Late outcome of spinal cord stimulation for unreconstructable and limb-threatening lower limb ischemia. Eur J Vasc Endovasc Surg 2007;33(6):717-724.
41. Klomp HM, Spincemaille GHJJ, Steyerberg EW, et al. Design issues of a randomized controlled clinical trial on spinal cord stimulation in critical limb ischemia. Eur J Vasc Endovasc Surg 1995;10(4):478-485.
42. Klomp HM, Steyerberg EW, van Urk H, Habbena, JDF. Spinal cord stimulation is not cost-effective for non-surgical management of critical limb ischemia. Eur J Vasc Endovasc Surg 2006;31(5):500-508.
43. Klomp HM, Steyerberg EW, Habbema JDF, et al. What is the evidence on efficacy of spinal cord stimulation in (subgroups of) patients with critical limb ischemia? Ann Vasc Surg 2009;23(3):355-363.
44. Klomp HM, Spincemaille GHJJ, Steyerberg EW, et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial. Lancet 1999;353(9158):1040-1044.
45. Ubbink DT, Spincemaille GHJJ, Prins MH, et al. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. J Vasc Surg 1999;30(2):236-244.
46. Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JDF. Pain and quality of life in patients with critical limb ischaemia: results of a randomized controlled multicentre study on the effect of spinal cord stimulation. Eur J Pain 2000;4(2):173-184.
47. Ubbink DT, Vermeulen H, Spincemaille GHJJ, et al. Systematic review and met-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg 2004;91(8):948-955.
48. Wang C, Schwaitzberg S, Berlinger E, et al. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch Surg 2003;138(3):272-279.
49. Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM 2004;97(7):385-395.
50. Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomized-controlled trial. Eur J Vasc Endovasc Surg 2003;25(6):513-518.
51. Strauss MB. Hyperbaric oxygen as an intervention for managing wound hypoxia: its role and usefulness in diabetic foot wounds. Foot Ankle Int 2005;26(1):15-18.
52. Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg 2005;92(1):24-32.
53. White CJ, Gray WA. Endovascular therapies for peripheral arterial disease: an evidence-based review. Circulation. 2007;116(19):2203-2215.